Abstract
Behcet’s disease (BD) is a chronic inflammatory condition involving several organs, such as skin, mucous membrane, eye, joint, intestine, lung and central nervous system. Ankylosing spondylitis (AS) is a prototype of seronegative spondyloarthropathy, and a chronic systemic inflammatory disorder of the axial skeleton, mainly affecting the sacroiliac joint and spine. In the latter, systemic complications may develop in addition to joint involvement. The coexistence of BD and AS has been rarely reported in the literature. The inclusion of BD among seronegative spondyloarthritides and whether sacroiliitis (SI) develops in BD are still being debated. We describe a 28-year-old man who has fulfilled the diagnostic criteria for BD and AS as well.
Keywords: Behcet’s disease, Ankylosing spondylitis, Sacroiliitis
INTRODUCTION
It is debatable that BD could be included among seronegative spondyloarthritides1,2). Some investigators have reported an increased prevalence of SI and AS in patients with BD, but others have found a prevalence that does not differ from that of the general population. The case described here was first diagnosed as ankylosing spondylitis. At that time, he had had recurrent oral ulcer. Several years later, painful scrotal ulcer and panuveitis that is a different clinical finding in AS have been developed.
CASE
A 28-year-old man presented with painful swelling of the right knee. He has been having recurrent oral ulcer for past ten years. Seven years ago, he has had pain in right heel, buttocks and lower back. The pain and stiffness in lower lumbar region and buttock was worse in the early morning and was improved with activity. Subsequently, painful swelling of the right knee developed. He was diagnosed to have AS at the University Medical Center. He had been treated with indomethacin, low-dose methotrexate and sulfasalazine during three years. After that, he withheld the medications by himself. Painful scrotal ulcer was occurred and painful swelling of right knee was developed again two years ago.
Physical examination revealed multiple aphthous ulcers on the buccal mucosa, papulopustular eruptions on the anterior chest, and scrotal ulcer. There were tenderness and swelling on the right knee. The chest expansion was 5 cm and the modified Shobber test was 15 cm. The pathergy test was negative. Hematological and biochemical tests were as follows: WBC 9200/mm3, hematocrit 40.1%, platelet 321000/mm3, total protein 7.2 g/dl, albumin 4.4 g/dl, AST 26 IU/L, ALT 35 IU/L, creatine kinase 76 IU/L. Erythrocyte sedimentation rate was 30 mm/hr and C-reactive protein was negative. Urinalysis and coagulation tests were normal. Rheumatoid factor, antinuclear antibody and antineutrophil cytoplasmic antibody were negative. Both HLA-B27 and B51 antigens were positive. Bilateral SI (right: grade 4, left: grade 3) was noted on plain pelvis radiograph (Figure 1) and T2-weighted magnetic resonance imaging (Figure 2).
Fig. 1.
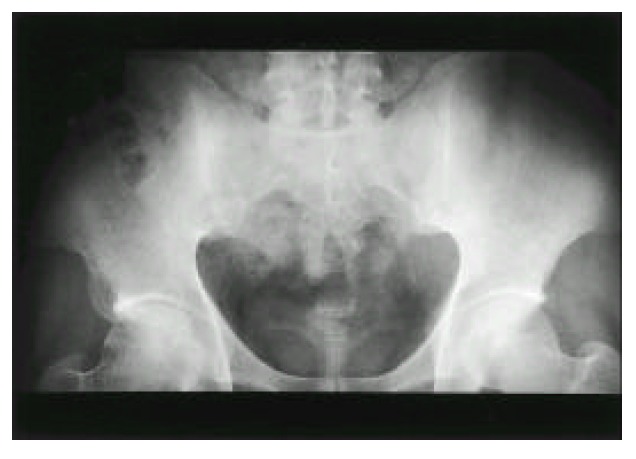
Plain pelvis radiograph shows the obliteration of joint space in both sacroiliac joints (right>left) and the juxta-articular bony sclerosis.
Fig. 2.
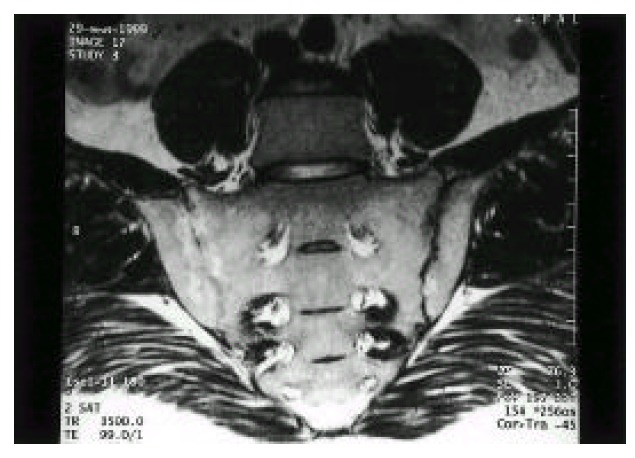
T2-weighted magnetic resonance imaging shows the increased signal intensity of subchondral marrow, and the obliteration of joint space in both sacroiliac joints (right>left).
Initially he was treated with indomethacin 50 mg/day, sulfasalazine 2g/day, methotrexate 7.5 mg/week and colchicine 1.2 mg/day. Because of persisting, painful swelling of the right knee, methotrexate was increased to 15 mg/week, and intra-articular injection of triamcinolone acetonide 40 mg was done. While being treated, recurrent acute iritis with hypopyon, posterior uveitis and papillitis in both eyes (Figure 3 & 4) were developed. Prednisolone 40 mg/day and cyclosporine 5 mg/kg/day were prescribed.
Fig. 3.
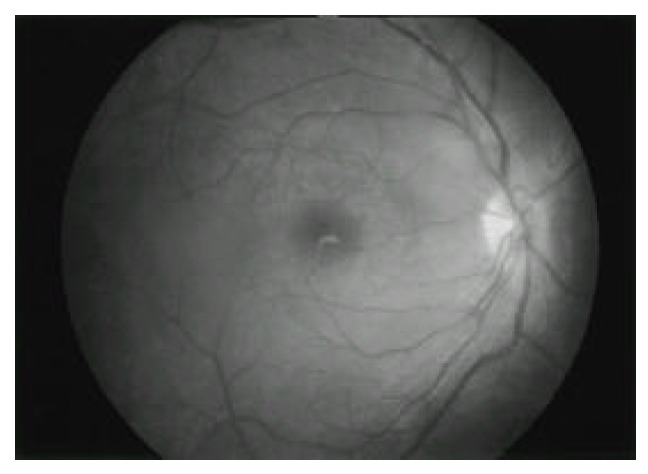
Fundus photograph of the right eye shows haziness due to posterior vitritis. There are elevation and blurring of the optic disc suggesting papillitis.
Fig. 4.
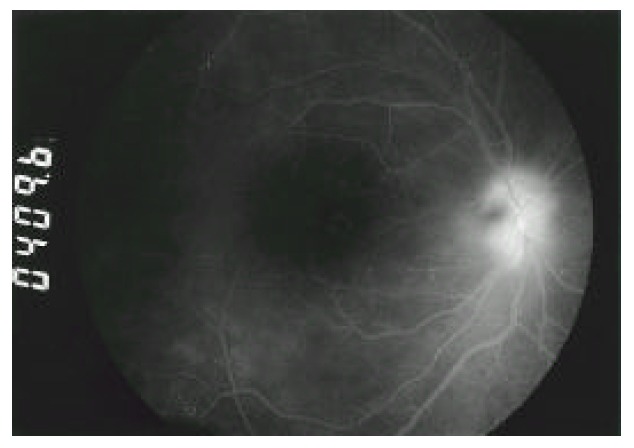
Fluorescein angiogram of the right eye reveals prominent leaking of optic disc and diffuse hyper-fluorescence due to posterior vitritis.
DISCUSSION
There are no specific diagnostic or laboratory tests for BD. The diagnosis of BD mainly depends upon the thorough history taking and clinical manifestations. The diagnosis of AS is also based on the clinical findings and the radiographic SI. Our case fulfilled the diagnostic criteria of BD by the International Study Group for Behcet’s Disease3) and modified New York criteria for AS4). Although there are some reports for a coexisting BD and AS5–8), it is unclear whether that occurs incidentally or overlaps like other connective tissue diseases.
Some investigators report a high prevalence of SI in patients with BD and therefore suggested that BD should be included in seronegative spondyloarthritides, whereas recent reports suggests no association between the two conditions. Dilsen and co-workers reported the highest values, 33 (10%) patients with AS and 112 (34%) with SI in 331 Turkish patients with BD9). Kahan et al. found AS in 2 (6.2%) of 32 patients with BD in France10). In Britain, Caporn et al reported erosive SI in 7 (50%) of 14 patients with BD11). However, other authors found no association between SI or AS and BD in Japan12), North America13), Iraq14), Britain15) and Turkey16). Yazici et al. reported only a single patient among 184 BD patients in Turkey17). It remains unclear why there are differences in prevalence between AS or SI and BD. Yazici and co-workers suggested that there was high observer variability in reading the anteroposterior radiographs of the sacroiliac joints18). Olivieri et al. suggested that computed tomography may reduce differences due to error in the radiological evaluation of the sacroiliac joints2). So far, there are no reports about association of SI or AS in patients with BD in Korea.
The course of eye involvement in BD and AS is so different. The HLA-B27-associated uveitis like AS usually involves the anterior uveal track and follows a benign course. However, the uveitis in BD involves both anterior and posterior uveal tracts and causes the loss of sight in 25 % of patients19). Whereas most patients with AS have HLA-B27 antigen, the relationship between HLA-B51 and BD appears to be in some racial populations. Both HLA-B27 and B51 (B5) antigens were more frequent in patients with coexisting BD and AS than in healthy controls9). The eye involvement in our case was anterior uveitis, posterior uveitis and retinal papillitis that are similar to the features in BD. He had both HLA-B27 and B51 antigens.
Until now the problem has been mainly limited to whether the frequency of SI and AS is greater in BD. In order to define the relationship between AS and BD, more reports and experience will be needed. Furthermore, case control studies should be done in different countries.
REFERENCES
- 1.Lee SK, Lee J. Behcet’s disease: A rheumatologic perspective. Yonsei Med J. 1997;38:395–400. doi: 10.3349/ymj.1997.38.6.395. [DOI] [PubMed] [Google Scholar]
- 2.Olivieri I, Salvarani C, Cantini F. Is Behcet’s disease part of the spondyloarthritis complex? J Rheumatol. 1997;24:1870–1872. [PubMed] [Google Scholar]
- 3.International Study Group for Behcet’s disease Criteria for diagnosis of Behcet’s disease. Lancet. 1990;335:1078–1080. [PubMed] [Google Scholar]
- 4.Van der Linden SM, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: A proposal for modification of the New York criteria. Anhritis Rheum. 1984;27:361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 5.Tosun M, Uslu T, Ibrahim Imamoglu H, Bahadir S, Erdolu S, Guler M. Coexisting ankylosing spondylitis and Behcet’s disease. Clin Rheumatol. 1996;15:619–620. doi: 10.1007/BF02238556. [DOI] [PubMed] [Google Scholar]
- 6.Olivieri I, Gemignani G, Busoni F, Pecori F, Camerini E, Trippi D, Pasero G. Ankylosing spondylitis with predominant involvement of the cervical spine in a woman with Behcet’s syndrome. Ann rheum Dis. 1988;47:780–783. doi: 10.1136/ard.47.9.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beiran I, Scharf J, Dori D, Miller B. A change in ocular involvement in a patient suffering from ankylosing spondylitis and Behcet’s disease. Eur J Ophthalmol. 1995;5:192–194. doi: 10.1177/112067219500500309. [DOI] [PubMed] [Google Scholar]
- 8.Olivieri I, Cantini F, Napoli V, Braccini G, Padula A, Pasero G. Seronegative spondyloarthropathy without spine involvement in Behcet’s syndrome. Clin Rheumatol. 1993;12:396–400. doi: 10.1007/BF02231587. [DOI] [PubMed] [Google Scholar]
- 9.Dilsen N, Konice M, Aral O. Why Behcet’s disease should be accepted as a seronegative arthritis. In: Lehner T, Barnes CG, editors. Recent Advances in Behcet’s disease. London: Royal Society of Medicine Services; 1986. pp. 281–284. [Google Scholar]
- 10.Kahan A, Amor B, Benhamou CL, Saporta L. Association du syndrome de Behcet et de la spondylarthrite ankylosante [Association of Behcet’s disease and ankylosing spondylitis] Ann Med Interne. 1982;133:573–575. [PubMed] [Google Scholar]
- 11.Caporn N, Higgs ER, Dieppe PA, Watt I. Arthritis in Behcet’s syndrome. Br J Radiol. 1983;56:87–91. doi: 10.1259/0007-1285-56-662-87. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu T, Ehrlich GE, Hayashi K. Behcet’s disease. Semin Anhritis Rheum. 1979;8:223–260. doi: 10.1016/0049-0172(79)90004-0. [DOI] [PubMed] [Google Scholar]
- 13.O’Duffy JD. Behcet’s disease. In: Kelley WN, Harris ED, Ruddy S, Sledge CB, editors. Textbook of Rheumatology. 3rd ed. Philadelphia: WB Saunders; 1989. pp. 1209–1214. [Google Scholar]
- 14.AI-Rawi ZS, Sharquie KE, Shalifa SJ, AI-Hadithi FM, Munir JJ. Behcet’s disease in Iraqi patients. Ann Rheum Dis. 1986;45:987–990. doi: 10.1136/ard.45.12.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamberlain MA. Behcet’s syndrome in 32 patients in Yorkshire. Ann Rheum Dis. 1977;36:491–499. doi: 10.1136/ard.36.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yurdakul S, Yazici H, Tzn Y, et al. The arthritis of Behcet’s disease: A prospective study. Ann Rheum Dis. 1983;42:505–515. doi: 10.1136/ard.42.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yazici H, Tuzlaci M, Yurdakul S. A controlled study of sacroiliitis in Behcet’s disease. Ann Rheum Dis. 1981;40:558–559. doi: 10.1136/ard.40.6.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yazici H, Turun M, zdogan H, Yurdakul S, Akinci A, Barnes CG. Observer variation in grading sacroiliac radiographs might be a cause of sacroiliitis reported in certain disease states. Ann Rheum Dis. 1987;46:139–145. doi: 10.1136/ard.46.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazleman BL. Rheumatic disorders of the eye and the various structures involved. Br J Rheumatol. 1996;35:258–268. doi: 10.1093/rheumatology/35.3.258. [DOI] [PubMed] [Google Scholar]


