Abstract
Background
Body temperature is usually regulated by opposing controls of heat production and heat loss. However, systemic administration of capsaicin, the pungent ingredient of hot peppers, facilitated heat production and heat loss simultaneously in rats. We recently found that the capsaicin-induced heat loss and heat production occur simultaneously and that the biphasic change in body temperature is a sum of transient heat loss and long-lasting heat production. Moreover, suppression of the heat loss response did not affect capsaicin-induced heat production and suppression of heat production did not affect capsaicin-induced heat loss. These observations suggest the independent peripheral mechanisms of capsaicin-induced thermal responses. Thus, the capsaicin-induced thermal responses apparently lack an integrated control.
Methods
Male Wistar rats were maintained at an ambient temperature of 24 ± 1°C on a 12 h on-off lighting schedule at least for two weeks before the experiments. They were anesthetized with urethane (1.5 g/kg, i.p.) and placed on a heating pad, which was kept between 29 and 30 °C. Skin temperature(Ts) was measured with a small thermistor, which was taped to the dorsal surface of the rat’s tail, to assess vasoactive changes indirectly. Colonic temperature(Tc) was measured with another thermistor inserted about 60 mm into the anus. O2 consumption was measured by the open-circuit method, and values were corrected for metabolic body size (kg0.75). Capsaicin (Sigma) was dissolved in a solution comprising 80% saline, 10% Tween 80, and 10% ethanol, and injected subcutaneously at a dose of 5 mg/kg. Each rat received a single injection of capsaicin because repeated administration of capsaicin renders an animal insensitive to the subsequent administration of capsaicin. Laminectomy was performed at the level of the first and second cervical vertebrae to expose the cervical spinal cord for sectioning. The brain was transected at 4-mm rostral from the interaural line with an L-shaped knife.
Results
After administration of capsaicin, O2 consumption increased from 13.5 ± 0.4 mL/min/kg0.75 at 0 min to a peak of 15.9 ± 0.4 mL/min/kg0.75 at 71 min and gradually declined but remained higher than the basal value until the end of the 4-h observation period. Ts also immediately increased from 27.7 ± 0.2 °C to 31.9 ± 0.3 °C at 39 min, and it returned to the baseline level within 90 min after the capsaicin administration. Tc initially decreased from 37.1±0.1 °C to 36.8 ± 0.2 °C at 43 min and then gradually increased over the baseline level and remained at 37.6 ± 0.2 °C until the end of the experiment, in spinalized rats, the capsaicin-induced increase in O2 consumption was largely attenuated, while the basal O2 consumption was similar to that of control rats. The basal Ts of spinalized rats was 32.4 ± 0.3 °C, which was higher than that of control rats. Capsaicin increased Ts by less than 1 °C, and Tc did not change after the capsaicin administration. O2 consumption of decerebrated rats was statistically higher than that of control rats after the injection of capsaicin. However, capsaicin did not increase Ts, showing a lack of a vasodilatory response. Decerebration between the hypothalamus and midbrain prevented the capsaicin-induced heat loss but not the heat production response.
Conclusion
These results show that the capsaicin-induced heat production and heat loss are controlled separately by the brainstem and by the forebrain, respectively, and suggest that the body temperature regulation is performed without an integrative center.
Keywords: Capsaicin, Body temperature, Heat production, Heat loss
INTRODUCTION
Mammals regulate their core body temperature within narrow limits by using their thermo-effectors, such as vasomotor, shivering or sweating, cooperatively and consistently, For example, animals exposed to a cold environment inhibit heat loss and facilitate heat production to keep their body temperature stable. Thus, heat loss and heat production work in the opposite direction and do not occur simultaneously in the normal body temperature regulation. Based on this fact, it is generally postulated that the putative thermoregulatory center provides an integrative function to establish homeothermy1).
Capsaicin stimulates a subpopulation of primary sensory neurons that are sensitive to warmth or noxious mechanical, chemical and thermal stimuli2–5). Thus, administration of capsaicin elicits a warm sensation at its threshold concentration and produces a burning pain at higher concentrations. Although capsaicin is nowadays known for its effect on the pain system, it has unique actions on body temperature. It is a common experience that an intake of capsaicin-containing spicy foods results in sweating and flushing of the face, which are the typical heat loss responses. In animals, capsaicin also induces coordinated heat loss responses, such as cutaneous vasodilatation, panting, salivation and increased body cooling behavior6,7). As a consequence of these heat loss responses, the body temperature decreases, although a long-lasting hyparthermia follows to exhibit a biphasic change8–10). The hyperthermia is a result of increased heat production: capsaicin stimulates adrenal sympathetic nerve activity, catecholamine release from the adrenal medulla and O2 consumption10–12). However, the capsaicin-induced heat production is not caused by the hypothermia. We recently found that the capsaicin-induced heat loss and heat production occur simultaneously and that the biphasic change in body temperature is a sum of transient heat loss and long-lasting heat production10). Moreover, suppression of the heat loss response did not affect capsaicin-induced heat production, and suppression of heat production did not affect capsaicin-induced heat loss. These observations suggest the independent peripheral mechanisms of capsaicin-induced thermal responses11,12). Thus, the capsaicin-induced thermal responses apparently lack an integrated control.
MATERIALS AND METHODS
Male Wistar rats, weighing 340–450 g, were maintained at an ambient temperature of 24 ± 1 °C on a 12 h on-off lighting schedule at least for two weeks before the experiments. The care of animals and all surgical procedures followed institutional and Japanese Physiological Society guidelines. They were anesthetized with urethane (1.5 g/kg, i.p.) and placed on a heating pad, which was kept between 29 and 30 °C. Their respiration was maintained through a tracheal cannula connected to an artificial respirator. Ts was measured with a small thermistor, which was taped to the dorsal surface of the rat’s tail, to assess vasoactive changes indirectly. Tc was measured with another thermistor inserted about 60 mm into the anus. O2 consumption was measured by the open-circuit method, and values were corrected for metabolic body size (kg0.75). Data were calculated at 1-min intervals and presented as mean ± SEM. A one-way ANOVA was used to determine significant changes in responses within groups. The Tukey test was used for multiple comparison. Significant differences were considered to have a p value of < 0.05.
Capsaicin (Sigma) was dissolved in a solution comprising 80% saline, 10% Tween 80 and 10% ethanol, and injected subcutaneously at a dose of 5 mg/kg. This dose was selected as being adequate to reveal the initial hypothermic and subsequent hyparthermic response in rats. Each rat received a single injection of capsaicin because repeated administration of capsaicin renders an animal insensitive to the subsequent administration of capsaicin.
Laminectomy was performed at the level of the first and second cervical vertebrae to expose the cervical spinal cord for sectioning. The spinal cord was transected with a pair of forceps, followed by suction with a blunt 21-G needle connected to a vacuum pump. This procedure resulted in removing a 1- to 2-mm segment of the spinal cord. Care was taken not to damage the cervical brown adipose tissue.
The brain was transected at 4-mm rostral from the interaural line with an L-shaped knife. The method of decerebration was described previously13). The level of decerebration was later confirmed by macroscopic examination of frozen sagittal sections of the brain.
RESULTS
Figure 1 shows the effects of capsaicin on O2 consumption, colonic temperature (Tc) and skin temperature (Ts) of urethane-anesthetized control rats. After administration of capsaicin, O2 consumption increased from 13.5 ± 0.4 mL/min/kg0.75 at 0 min to a peak of 15.9 ± 0.4 mL/min/kg0.75 at 71 min and gradually declined but remained higher than the basal value until the end of the 4-h observation period (Figure 1A). Ts also immediately increased from 27.7 ± 0.2 °C to 31.9 ± 0.3 °C at 39 min (Figure 1C), suggesting skin vasodilatation, and it returned to the baseline level within 90 min after the capsaicin administration. Tc initially decreased from 37.1 ± 0.1 °C to 36.8 ± 0.2 °C at 43 min and then gradually increased over the baseline level and remained at 37.6 ± 0.2 °C until the end of the experiment (Figure 1B). The results confirmed the simultaneous activation of heat production and heat loss after capsaicin administration10).
Figure 1.
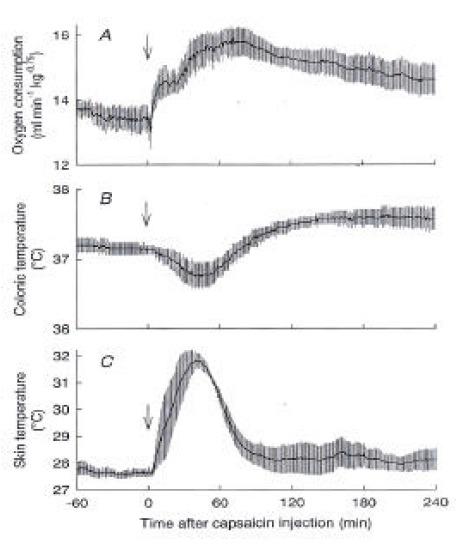
Responses of control rats to capsaicin. (A) O2 consumption, (B) colonic temperature, and (C) skin temperature recorded from sham-operated control rats (n=4) after administration of capsaicin (5 mg/kg s.c.) are shown. The biphasic change in colonic temperature reflects the sum of the initial heat loss response, suggested by an increase in skin temperature, and long-lasting heat production, shown by an increase in O2 consumptiom. These rats were treated with either sham spinal transection or sham decerebration. In two rats, the cervical vertebrae were exposed, but laminectomy was not made. In two other rats, a slit was made in the skull but the knife was not inserted into the brain. Data taken from these four rats were pooled. Arrows, time of capsaicin injection.
We examined the involvement of the brain in the capsaicin-induced thermoregulatory responses by spinal transection and decerebration. Figure 2 illustrates the levels of brain transections. In spinalized rats, the capsaicin-induced increase in O2 consumption was largely attenuated, while the basal O2 consumption was similar to that of control rats (Figure 3A). O2 consumption showed a small peak of 14.2 ± 0.1 mL/min/kg0.75 at 2 min and then slowly declined to the baseline level. The basal Ts of spinalized rats was 32.4 ± 0.3 °C, which was higher than that of control rats, suggesting steady vasodilatation (Figure 3C). Capsaicin increased Ts by less than 1 °C, and Tc did not change after the capsaicin administration (Figure 3B).
Figure 2.

Illustration of the rat brain (0.5 mm lateral to the midline) showing the levels of transection. Decerebration was defined as the transection between the hypothalamus and midbrain. Spinal transection was made at the level of the first and second cervical vertebrae.
Figure 3.
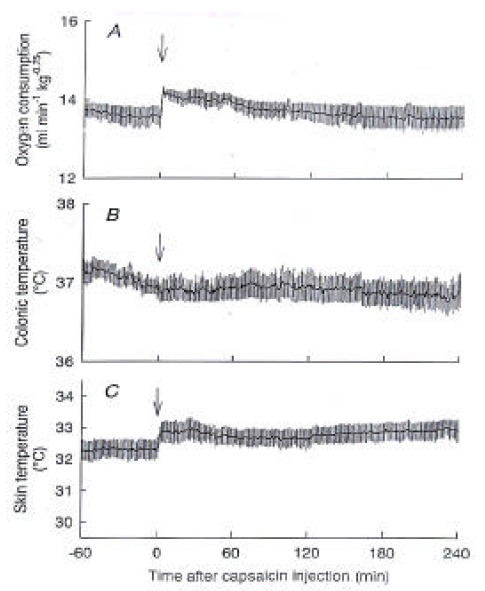
Responses of spinalized rats to capsaicin. (A) O2 consumption, (B) colonic temperature, and (C) skin temperature recorded from spinalized rats (n=4) after capsaicin treatment are shown. The capsaicin-induced responses were largely, but not completely, blocked by the spinal transection. Arrows, capsaicin injection.
In decerebrated rats that were transected between the hypothalamus and midbrain, capsaicin increased O2 consumption from 14.5 ± 0.5 mL/min/kg0.75 to a peak of 18.1 ± 0.9 mL/min/kg0.75 at 46 min. O2 consumption of decerebrated rats was statistically higher than that of control rats during 21–58 min and 106–240 min after the injection of capsaicin (Figure 4A). However, capsaicin did not increase Ts, showing a lack of a vasodilatory response (Figure 4C). Consequently, decerebrated rats did not show the hypothermic period and monophasically increased Tc by 1.2 °C until the end of the experiment (Figure 4B).
Figure 4.
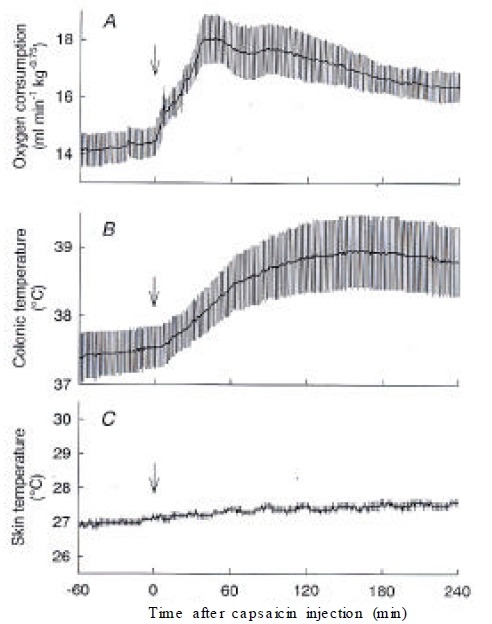
Responses of decerebrated rats to capsaicin. (A) O2 consumption, (B) colonic temperature, and (C) skin temperature recorded from decerebrated rats (n=4) after capsaicin treatment are shown. Note the lack of capsaicin-induced change in skin temperature. The maximal increase in O2 consumption was higher than that of the control rats. Colonic temperature increased without the initial hypothermic period seen in control rats. Arrows, capsaicin injection.
DISCUSSION
The present study demonstrated that administration of capsaicin evoked only small increases in O2 consumption and Ts in spinalized rats. Thus, most capsaicin-induced responses require the brain, and spinal or peripheral mechanisms caused the residual small responses. Capsaicin promotes a release of vasodilatory peptides from capsaicin-sensitive nerve terminals in the periphery14). Thus, the small increase in Ts in spinalized rats might be caused by this peripheral mechanism. Capsaicin also stimulated O2 consumption of isolated rat hindlimb preparation15), and local injection of capsaicin into the thoracic interspinous tissue enhanced the adrenal nerve activity and catecholamine secretion16). Thus, these peripheral mechanisms could cause the small increases in O2 consumption seen in the spinalized rats.
The present study also demonstrated that decerebration between the hypothalamus and midbrain prevented the capsaicin-induced heat loss response and hypothermia but did not attenuate the heat production response. Because the forebrain was isolated from the rest of the body in this preparation, it is critically involved in the mechanism of capsaicin-induced heat loss. The preoptic area of the hypothalamus is located in the forebrain, contains inherent thermosensitive neurons and is considered to be the principal integrative site of thermoregulation17). Systemic and local application of capsaicin excites warm-sensitive neurons in the preoptic area18,19). Generally, excitation of warm-sensitive neurons activates heat loss mechanisms1,17). Thus, the response of warm-sensitive neurons to capsaicin is favorable in explaining the capsaicin-induced heat loss.
On the other hand, decerebrated rats significantly increased O2 consumption and Tc without the initial hypothermic period after capsaicin administration. Thus, the forebrain is not critically involved in the capsaicin-induced heat production. Because the heat production response was largely attenuated in the spinalized rats, some regions in the brainstem, including the midbrain, pons, and medulla oblongata are responsible for this response. Therefore, the capsaicin-induced heat production and heat loss are controlled separately by the brainstem and by the forebrain, respectively.
It is worth noting that the decerebrated rats did not show any heat loss response, whereas the spinalized rats showed a slight increase in Ts. These results suggest that the brainstem exerts an inhibitory influence on the capsaicin-induced spinal mechanism of vasodilatation and that spinalization probably removed this inhibition. Moreover, the increase in capsaicin-induced O2 consumption of decerebrated rats was higher than that of control rats. This result suggests that the forebrain has an inhibitory mechanism for heat production20–22) and that the decerebration released this inhibition.
Capsaicin stimulates nociceptors and the pain system2–5), which results in various stress-related thermoregulatory responses. For example, hypermetabolism occurs during restraint-stress, which is accompanied by a large increase in plasma adrenaline and can be attenuated by adrenalectomy or by chemical sympathectomy via administration of 6-hydroxydopamine23,24). Accordingly, it is possible that capsaicin activated the system mediating the stress hyparthermia. The pain-and-stress system activates the hypothalamo-pituitary-adrenal axis and associated responses, which in turn may affect heat production. However, it is unlikely that these forebrain mechanisms play the major role in the capsaicin-induced heat production, because decerebration did not prevent this response in the present study. Thus, the brainstem mechanisms are responsible for the capsaicin-induced thermogenesis. Capsaicin-sensitive sensory neurons probably send afferent information to the brainstem, which integrates these signals and activates the spinal heat production mechanisms. Alternatively, the ventrolateral medulla in the brainstem contains capsaicin-sensitive structure, which is involved in the temperature response to endotoxin25); and thus it may participate in the capsaicin-induced thermogenesis. This area also contains premotor neurons innervating the sympathoadrenal preganglionic neurons in the spinal cord26,27). Thus, the medullary presympathetic neurons might lead to excitation of the adrenal sympathetic activity and catecholamine release, resulting in an increase in heat production.
In summary, capsaicin activated the forebrain heat loss mechanism and the brainstem heat production mechanism simultaneously. Based on these results, we presume that these capsaicin-induced thermoregulatory mechanisms are composed of basically separate neural circuits, although the forebrain exerts an inhibitory influence on the brainstem heat production mechanism. Generally, various thermo-effector systems do not have the identical temperature threshold and are considered to be composed of separate and independent neural circuits28–30). Accordingly, the apparent coordinated control of body temperature in the normal condition does not necessarily indicate the evidence of integrative function of the central thermoregulation. Although multiple parallel thermoregulatory systems behave consistently, as if the integrative center coordinated the whole thermoregulatory mechanisms in most physiological situations, the effects of capsaicin suggest that heat production and heat loss work independently.
REFERENCES
- 1.Bligh J. In: In Thermoreception and Temperature Regulation. Bligh J, Voigt K, editors. Springer-Verlag; Berlin: 1990. pp. 163–173. [Google Scholar]
- 2.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43(2):143–201. [PubMed] [Google Scholar]
- 3.Szolcsanyi J. Capsaicin The Study of Pain. London: JN Wood, Ed. Academic Press; 1993. [Google Scholar]
- 4.Szallasi A, Nilsson S, Farkas Szallasi T, Blumberg PM, Hokfelt T, Lundberg JM. Vanilloid (capsaicin) receptors in the rat: distribution in the brain, regional differences in the spinal cord, axonal transport to the periphery, and depletion by systemic vanilloid treatment. Brain Res. 1995;703(1–2):175–183. doi: 10.1016/0006-8993(95)01094-7. [DOI] [PubMed] [Google Scholar]
- 5.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 6.Szolcsanyi J. In: Handbook of Experimental Pharmacology. Milton A.S, editor. Berlin: Springer-Verlag; 1982. [Google Scholar]
- 7.Hori T. Capsaicin and central control of thermoregulation. Pharmacol Ther. 1984;26(3):389–416. doi: 10.1016/0163-7258(84)90041-x. [DOI] [PubMed] [Google Scholar]
- 8.Jancso Gabor A, Szolcsanyi J, Jancso N. Irreversible impairment of thermoregulation induced by capsaicin and similar pungent substances in rats and guinea-pigs. J Physiol Lond. 1970;206(3):495–507. doi: 10.1113/jphysiol.1970.sp009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szikszay M, Obal F, Jr, Obal F. Dose-response relationships in the thermoregulatory effects of capsaicin. Naunyn Schmiedebergs Arch Pharmacol. 1982;320(2):97–100. doi: 10.1007/BF00506307. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi A, Osaka T, Namba Y, Inoue S, Lee TH, Kimura S. Capsaicin activates heat loss and heat production simultaneously and independently in rats. Am J Physiol. 1998;275(1 Pt 2):R92–98. doi: 10.1152/ajpregu.1998.275.1.R92. [DOI] [PubMed] [Google Scholar]
- 11.Kawada T, Watanabe T, Takaishi T, Tanaka T, Iwai K. Capsaicin-induced beta-adrenergic action on energy metabolism in rats: influence of capsaicin on oxygen consumption, the respiratory quotient, and substrate utilization. Proc Soc Exp Biol Med. 1986;183(2):250–256. doi: 10.3181/00379727-183-42414. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T, Kawada T, Kurosawa M, Sato A, Iwai K. Adrenal sympathetic efferent nerve and catecholamine secretion excitation caused by capsaicin in rats. Am J Physiol. 1988;255(1 Pt 1):E23–27. doi: 10.1152/ajpendo.1988.255.1.E23. [DOI] [PubMed] [Google Scholar]
- 13.Osaka T, Yoshimatsu H, Kannan H, Yamashita H. Activity of hypothalamic neurons in conscious rats decreased by hyperbaric environment. Brain Res Bull. 1989;22(3):549–555. doi: 10.1016/0361-9230(89)90110-x. [DOI] [PubMed] [Google Scholar]
- 14.Maggi CA, Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol. 1988;19(1):1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- 15.Cameron Smith D, Colquhoun EQ, Ye JM, Hettiarachchi M, Clark MG. Capsaicin and dihydrocapsaicin stimulate oxygen consumption in the perfused rat hindlimb. Int J Obes. 1990;14(3):259–270. [PubMed] [Google Scholar]
- 16.Budgell B, Sato A, Suzuki A, Uchida S. Responses of adrenal function to stimulation of lumbar and thoracic inte rspinous tissues in the rat. Neurosci Res. 1997;28(1):33–40. doi: 10.1016/s0168-0102(97)01173-5. [DOI] [PubMed] [Google Scholar]
- 17.Hori T. An update on thermosensitive neurons in the brain: from cellular biology to thermal and non-thermal homeostatic functions. Jpn J Physiol. 1991;41(1):1–22. doi: 10.2170/jjphysiol.41.1. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama T, Yamamoto K, Ishikawa Y, Imai K. Effects of preoptic thermal stimulation on the ventromedial hypothalamic neurons in rats. Neurosci Lett. 1981;26(2):177–181. doi: 10.1016/0304-3940(81)90345-1. [DOI] [PubMed] [Google Scholar]
- 19.Hori T, Shibata M, Kiyohara T, Nakashima T, Asami A. Responses of anterior hypothalamic-preoptic thermosensitive neurons to locally applied capsaicin. Neuropharmacology. 1988;27(2):135–142. doi: 10.1016/0028-3908(88)90162-1. [DOI] [PubMed] [Google Scholar]
- 20.Shibata M, Hori T, Kiyohara T, Nakashima T. Facilitation of thermoregulatory heating behavior by single cortical spreading depression in the rat. Physiol Behav. 1983;31(5):561–566. [PubMed] [Google Scholar]
- 21.Shibata M, Hori T, Nagasaka T. Effects of single cortical spreading depression on metabolic heat production in the rat. Physiol Behav. 1985;34(4):563–567. doi: 10.1016/0031-9384(85)90049-6. [DOI] [PubMed] [Google Scholar]
- 22.Chen XM, Hosono T, Yoda T, Fukuda Y, Kanosue K. Efferent projection from the preoptic area for the control of non-shivering thermogenesis in rats. J Physiol Lond. 1998;512(Pt 3):883–892. doi: 10.1111/j.1469-7793.1998.883bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buhler HU, da Prada M, Haefely W, Picotti GB. Plasma adrenaline, noradrenaline and dopamine in man and different animal species. J Physiol Lond. 1978;276:311–320. doi: 10.1113/jphysiol.1978.sp012235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagasaka T, Hirata K, Sugano Y, Shibata H. Heat balance during physical restraint in rats. Jpn J Physiol. 1979;29(4):383–392. doi: 10.2170/jjphysiol.29.383. [DOI] [PubMed] [Google Scholar]
- 25.Koulchitsky SV. Are the capsaicin-sensitive structures of ventral medulla involved in the temperature response to endotoxin in rats? Neurosci Lett. 1998;244(2):112–114. doi: 10.1016/s0304-3940(98)00128-1. [DOI] [PubMed] [Google Scholar]
- 26.Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res. 1989;491(2):274–296. doi: 10.1016/0006-8993(89)90063-2. [DOI] [PubMed] [Google Scholar]
- 27.Wesselingh SL, Li YW, Blessing WW. PNMT-containing neurons in the rostral medulla oblongata (C1, C3 groups) are transneuronally labelled after injection of herpes simplex virus type 1 into the adrenal gland. Neurosci Lett. 1989;106(1–2):99–104. doi: 10.1016/0304-3940(89)90209-7. [DOI] [PubMed] [Google Scholar]
- 28.Satinoff E. Neural organization and evolution of thermal regulation in mammals. Science. 1978;201(4350):16–22. doi: 10.1126/science.351802. [DOI] [PubMed] [Google Scholar]
- 29.Hensel H. Thermoreception and Temperature Regulation. London: Academic Press; 1981. [PubMed] [Google Scholar]
- 30.Kazuyuki K, Hosono T, Zhang YH, Chen XM. Neuronal networks controlling thermoregulatory effectors. Prog Brain Res. 1998;115:49–62. doi: 10.1016/s0079-6123(08)62029-4. [DOI] [PubMed] [Google Scholar]


