Table 1.
Development of the catalytic aminofluorination reaction[a]
| Entry | Catalyst | Solvent | Yield [%][b] |
|---|---|---|---|
| 1 | [Cu(MeCN)4]BF4 | CH2Cl2 | 65 |
| 2 | [Pd(MeCN)4](BF4)2 | CH2Cl2 | 50 |
| 3 | [Ag(MeCN)4]BF4 | CH2Cl2 | 46 |
| 4 | AgBF4 | CH2Cl2 | 58 |
| 5 | CuCl | CH2Cl2 | 0 |
| 6 | Zn(BF4)2⋅x H2O | CH2Cl2 | 75 |
| 7 | Zn(BF4)2⋅x H2O | THF | 40 |
| 8 | Zn(BF4)2⋅x H2O | PhCH3 | 63 |
| 9 | Zn(BF4)2⋅x H2O | CH3CN | <5 |
| 10[c] | Zn(BF4)2⋅x H2O | CH2Cl2 | 0 |
| 11 | – | CH2Cl2 | 0 |
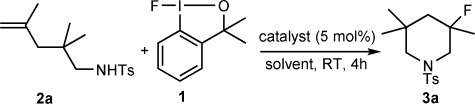
[a] Reaction conditions: 0.1 mmol of 2 a, Zn(BF4)2⋅XH2O (5 mol %) and 1 (1.1 equiv) was reacted in CH2Cl2 (0.5 mL) at RT. [b] Yield of the isolated product. [c] Fluoroiodine 1 was not added. THF=tetrahydrofuran.
