Table 3.
Fluorocyclization by oxy- and carbofluorination using 1[a]
| Entry | Substrate | Method | t [h] | Product | Yield [%][b] |
|---|---|---|---|---|---|
| 1 | 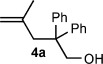 |
A | 2 | 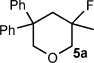 |
62 |
| 2 | 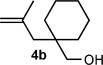 |
A | 1 | 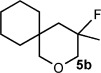 |
65 |
| 3 | 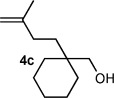 |
A | 1 | 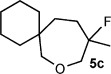 |
60 |
| 4 | 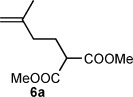 |
B | 8 | 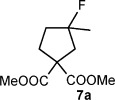 |
55 |
| 5 | 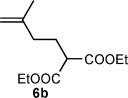 |
B | 8 | 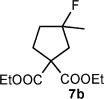 |
60 |
| 6[c] | 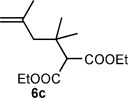 |
B | 8 | 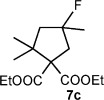 |
76 |
| 7 | 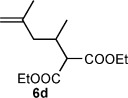 |
B | 4 | 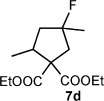 |
60 (2:1)[d] |
[a] Method A: 0.3 mmol of 4, Zn(BF4)2⋅x H2O (5 mol %) and 1 (1.1 equiv) was reacted in CH2Cl2 (0.5 mL) at RT; Method B: 0.3 mmol of 6, [Cu(MeCN)4]BF4 (10 mol %) and 1 (1.5 equiv) was reacted in CH2Cl2 (0.5 mL) at 40 °C. [b] Yield of the isolated product. [c] The reaction was performed at RT. [d] The ratio of diastereomers was determined by 19F NMR analysis of the crude reaction mixture.
