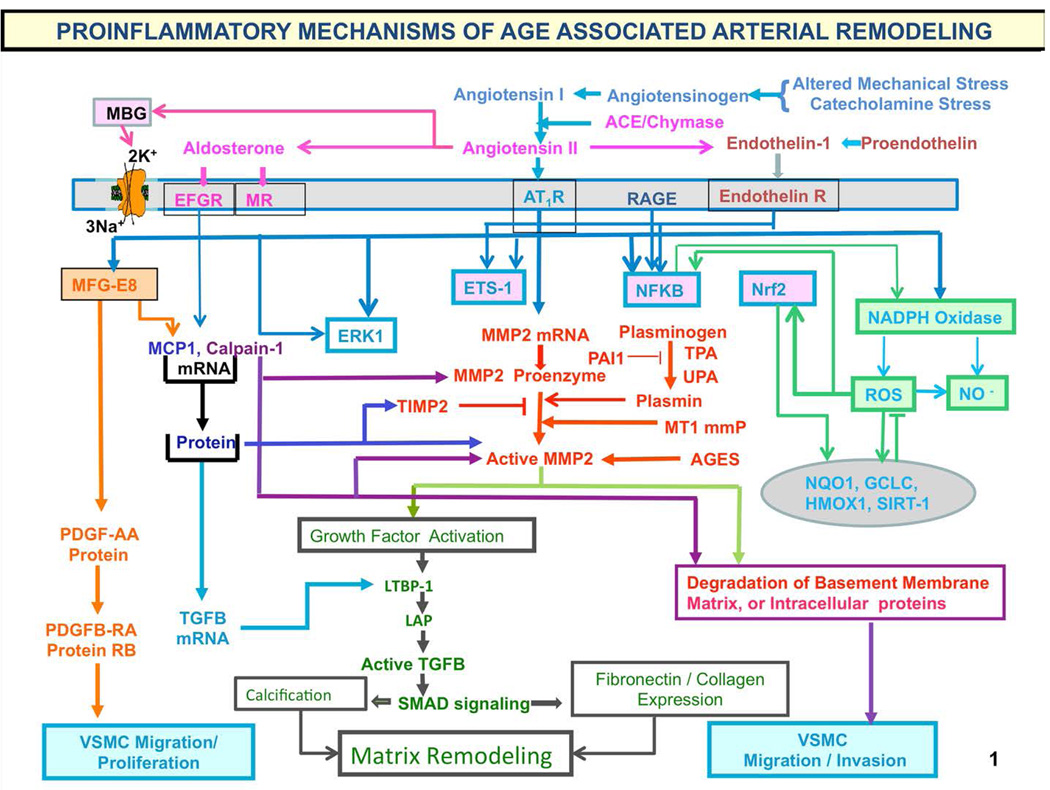
Figure 9. Proinflammatory mechanisms of age-associated arterial remodeling.
Processes that lead to cellular and matrix structural and functional age-associated changes of the arterial wall are driven by a proinflammatory microenvironment and low-grade inflammation, mediated by mechanical and humoral factors. These processes are driven by oxidative stress. Abbreviations: AAASP, age-associated arterial secretory phenotype; ACE, angiotensin converting enzyme; Ang II, angiotensin II; AT1R, angiotensin II type 1 receptor; AGE, advanced glycation endproducts; ECM, extracellular matrix; ET-1, endothelin-1; ETA, endothelin-1 receptor A; Ets-1, v-ets erythroblastosis virus E26 oncogene homolog 1; LAP, latency-associated peptide; LTBP-1; latent transforming growth factor (TGF)-binding protein-1 (LTBP-1) and MCP-1, monocyte chemoattractant protein-1; MFG-E8, milk fat globule epidermal growth factor-8; MMP, matrix metalloprotease; MR, aldosterone/mineralocorticoid receptor; NF-kB, nuclear factor k light-chain-enhancer of activated B cells; Nrf-2, NF-E2-related factor 2; NO, nitric oxide; PAI, plasminogen activator inhibitor; PDGF, platelet-derived growth factor; RAGE, receptor for AGE; ROS, reactive oxygen species; TGF-b1, transforming growth factor b1; t-PA/u-PA, tissue-type/plasminogen-type plasminogen activators; VMSC, vascular smooth muscle cell.
The chronic proinflammatory profile within aged central arteries is driven by alterations in signaling systems that include Ang II signaling via its receptor AT1, as well as MR, and ET-1/ETA and RAGE signaling. NF-kB and Ets-1 are activated within, whereas other factors such as Nrf-2 are reduced. Downstream signaling molecules include MFG-E8, MMPs, calpain-1, MCP-1, and TGF-b1. Calpain-1, MMPs, TGF-b1, NADPH oxidase activation increases and NO bioavailability decreases. VSMCs from old arteries secrete increased amounts of MFG-E8, MCP-1, MMP-2, and TGF-b1 inducing VSMC proliferation, migration, secretion, senescence, extracellular arterial matrix deposition becomes markedly altered with aging. Disruption of the endothelium, intima-media thickening, arterial amyloidosis, fibrosis, calcification, elastin fracture, and matrix glycoxidative modifications are consequences of the enhanced signaling via the depicted receptor signaling cascades and can lead to changes in arterial clinical phenotype that can be measured non-invasively in humans. Our body's initial response to stress is moderated by increased adrenergic signaling; the downstream receptor signaling cascade results in increased activation of renin-angiotensin-aldosterone, and endothelial dysfunction, mechanisms that our body utilizes to respond to chronic stress. Importantly, this stress defense is not executed by “professional” inflammatory cells (i.e. white blood cells) but by endothelial and VSM cells that shift their phenotypes to produce inflammatory cytokines. (adapted from Ref. 8)