Fig. 1. Proposed asymmetric hydroamination of unactivated internal olefins to access enantioenriched branched aliphatic amines.
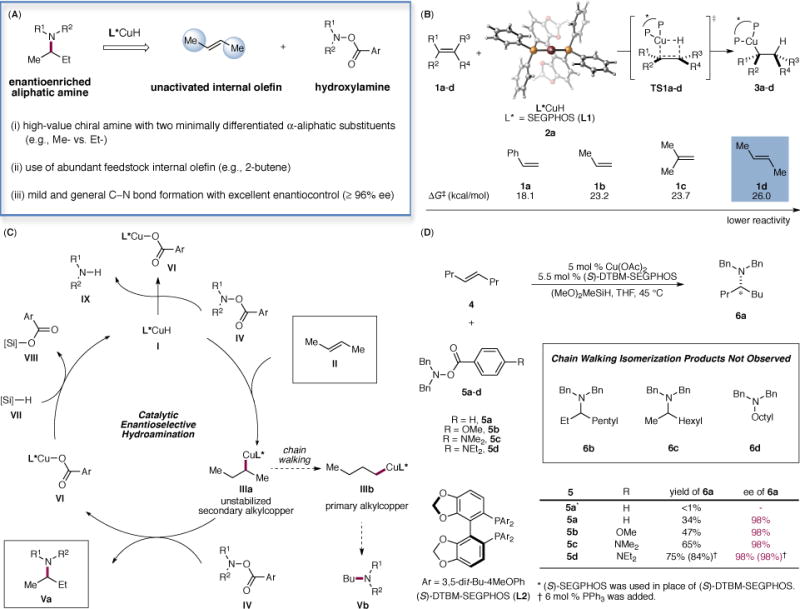
(A) Advantageous properties of the reaction profile. (B): DFT-calculated activation barriers for the hydrocupration of olefins 1a–d. Energies are computed at the M06/SDD-6-311+G(d,p)/SMD(THF) level with geometries optimized at the B3LYP/SDD-6-31G(d) level. (C): Proposed catalytic cycle. (D): Optimization studies. Reactions were performed using 4 (0.60 mmol), 5 (0.20 mmol), (MeO)2MeSiH (0.60 mmol), Cu(OAc)2 (5 mol %), L (5.5 mol %) in THF (1.0 M) at 45 °C for 36 h. Yields were determined by GC analysis using dodecane as the internal standard.
