Abstract
Aims
Novel therapeutic approaches for the treatment of malignant peripheral nerve sheath tumors (MPNSTs) are critically needed. Tyrosine kinase receptors are commonly deregulated in cancer and constitute attractive targets. We assessed the protein expression level of a panel of ‘drugable’ TKRs in a relatively large cohort of human plexiform and MPNST surgical specimens.
Methods and Results
Immunohistochemistry for HER2, PDGFRA, PDGFRB, KIT, IGF-1R, MET, and AXL was performed on an MPNST tissue microarray, yielding data from 99 tumors (plexiform/atypical neurofibroma = 26 and MPNST =73). PDGFRA, PDGFRB, MET, IGFR, and AXL were found to be highly expressed in human MPNST and all but AXL were significantly higher in MPNST as compared to neurofibroma. No HER2 expression was found. KIT expression in tumor cells was uncommon, but highlighted mast intratumoral cells in both neurofibroma and MPNST.
Conclusions
Several TKRs were overexpressed in MPNSTs, exhibiting tumor-to-tumor heterogeneity. When designing future MPNST clinical trials, pre-treatment molecular analysis may help in ‘smart’ patient selection. Furthermore, utilizing single compounds blocking multiple TKRs or therapeutic combinations could constitute a superior anti-MPNST treatment approach.
Malignant peripheral nerve sheath tumors (MPNSTs) are aggressive malignancies developing sporadically (~50%) or in neurofibromatosis type-1 contexts (NF1; ~50%).1. Surgical resection is the only curative intervention for these highly chemoresistant tumors 1. Unfortunately many MPNSTs progress during treatment; inoperable disease is generally lethal, 2, 3 mandating improved molecular-targeted MPNST therapeutic strategies.
While Nf1 protein loss with enhanced RAS pathway signaling activation may contribute to initial transformation, 1,2 additional genetic/epigenetic molecular aberrations are required for tumorigenesis and MPNST progression.6 Tyrosine kinase receptor (TKR) over-expression and deregulated signaling occur in many malignancies, inducing activation of signaling pathways mediating tumor progression.3 Several of these receptors (e.g. EGFR, HER2, KIT) have been successfully utilized as anti-cancer therapeutic targets, providing strong rationales for blocking cancer-type specific deregulated TKRs. Several TKRs may be over-expressed in MPNST.4 However, due to tumor rarity, such insights are generally made using small MPNST sample cohorts. With an overarching goal of developing MPNST clinical trials using molecularly-targeted therapies, we evaluated protein expression levels of multiple TKRs in a large MPNST cohort assembled on a previously described tissue microarray (TMA).2
HER2 (RTK class I)
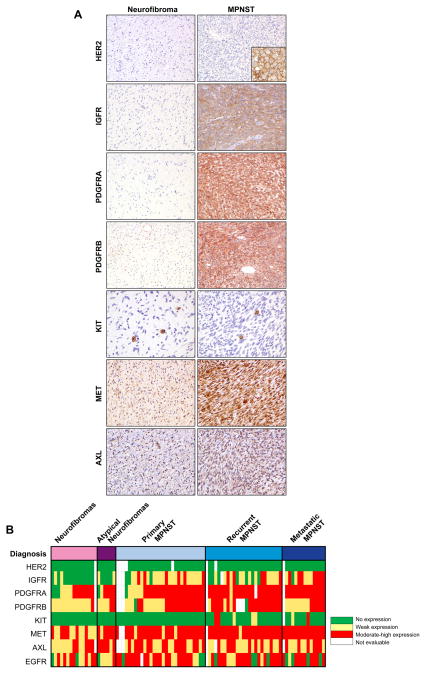
EGFR was previously shown to be commonly over-expressed in MPNST.1,5 Here, we evaluated HER2, another class-I member. Interestingly, in contrast to previous published data,5 no HER2 expression was found in MPNST or their benign neurofibroma counterparts.
IGFR (RTK class II)
Most neurofibromas (n=16; 80.0%) did not express IGF-1R; the remainder demonstrating only low levels. In contrast, 51 MPNSTs expressed IGF-1R; 18 of these (24.47%) exhibiting moderate-to-high intensity (p<0.001; Fig 1, Table 1).
Figure 1. “Targetable” tyrosine kinase receptors expression in MPNST.
A, Immunohistochemical staining of representative neurofibromas (left column) and MPNST samples (right column); HER2 (Inset on the bottom right side represents a positive control for HER2 staining, i.e. breast carcinoma), IGFR, PDGFRA, PDGFRB, KIT, MET and AXL. B, Heat map demonstrating the relative levels of expression of the respective receptors. The vertical columns represent individual samples. The horizontal rows represent the diagnosis (top) and tyrosine kinase receptor assayed (below). The colors in each cell indicate the expression level of a particular tyrosine kinase in a individual sample.
Table 1.
TKRs expression in plexiform/atypical neurofibroma and MPNST
| Neurofibroma (N=21) | MPNST (N=68) | ||
|---|---|---|---|
|
| |||
| Biomarkers* | mean intensity (±Std) | mean intensity (±Std) | p-value |
| IGFR | 0.2 (±0.41) | 1.16 (±0.77) | <0.001 |
| PDGFRA | 1.55 (±1.1) | 2.44 (±0.74) | <0.001 |
| PDGFRB | 1.15(±0.37) | 1.83 (±0.85) | 0.001 |
| KIT | 0.00 (NA)*** | 0.16 (±0.54) | 0.13 |
| KIT positive mast cells** | 6.48 (±9.89) | 4.09 (±8.53) | 0.016 |
| MET | 1.67 (±0.73) | 2.55 (±0.61) | <0.001 |
| AXL | 1.60 (±0.68) | 1.56 (±0.64) | 0.862 |
HER2 staining was negative in all samples tested
Scoring represents average number of KIT positive mast cells per X200 magnification field (±SD)
KIT was negative in every neurofibroma sample
PDGFRs (RTK class III)
All MPNSTs at least moderately expressed PDGFRA. PDGFRB was expressed in 48 (70.6 %) MPNST cases, with 37 (54%) samples demonstrating at least moderate intensity (Fig 1). Similarly, 16 and 20 (76% and 95%) neurofibroma cases were found to express PDGFRA and B, respectively; 11 and 3 samples (52% and 14% respectively) expressing moderate-to-high levels. The difference in PDGFRA and B expression intensity between MPNST and neurofibroma was highly significant (Table 1). Of potential relevance, PDGFRA expression was significantly higher in metastatic lesions compared to localized MPNST (p=0.001); no such differences were observed for PDGFRB.
KIT (RTK class III)
Only 7 MPNST samples (10%) exhibited tumor cell-associated KIT expression; moderate-to-high levels were limited to 3 specimens (Fig 1). No neurofibromas expressed KIT (Table 1). As expected, mast cells within tumor tissues were KIT positive, thereby enabling their quantization (Table 1, Fig 1). Interestingly, a statistically significant lower mast cell number was found in MPNSTs as compared to neurofibromas (mean of 4 versus 6.5 per X200 field, respectively; p=0.016).
MET (RTK class VI)
MET expression was found in 21 (100%) cases of neurofibromas; moderate-to-high expression was observed in 11 (52%) samples. All MPNSTs expressed MET; low expression was observed in 4 (6%) and moderate-to-high in 61 (90%; Fig 1) cases. Taken together, MPNST expressed a significantly higher level of MET compared to plexifom neurofibroma (p<0.001; Table 1).
AXL (RTK class IX)
Twenty (95%) of neurofibromas expressed AXL; moderate-to-high expression was found in 10 samples (48%; Fig 1). Similarly, 62 (91%) of MPNST specimens expressed AXL; moderate-to-high expression was found in 30 (44%).
No statistical difference in AXL expression level was observed between neurofibroma and MPNST (Table 1), suggesting that AXL deregulation might be an early event. No significant difference in expression of any of the RTKs evaluated was noted between NF1-associated and sporadic MPNST and only PDGFRA expression levels, as described above, were found to differentiate between localized versus metastatic lesions.
In conclusion, mapping TKR expression in human plexiform neurofibroma and MPNST, we have identified several over-expressed ‘drugable’ target candidates for further preclinical investigation. These results also highlight several MPNST-related factors of potential translational/clinical importance. First, MPNSTs are highly heterogeneous with significant TKR inter-tumoral expression variability. Consequently, the ‘one size fits all’ therapeutic approach is not relevant when designing new ‘smart’ or selective clinical trials and should be replaced with individualized treatment strategies. Molecular MPNST analysis to inform treatment decisions may help connect patient subgroups with specific therapeutic strategies. Secondly, accumulating experience with molecularly targeted therapies suggest that most cancers will defy single-molecule-targeted therapy, showing either transient or no benefits.6 An MPNST-relevant example is the recent clinical phase II evaluation of the EGFR inhibitor erlotinib, demonstrating no objective responses in any of 24 relapsed MPNST patients in contrast to a large body of pre-clinical data suggesting an important EGFR role in MPNST tumorigenesis and progression.7 Regarding TKR targeting, data suggests redundancy among different receptors such that activation of one could compensate for blockade of another (e.g. EGFR and MET).8 Based on our study (see Fig 1 heatmap), it is quite apparent that a single MPNST may highly express two or more different TKRs. Consequently, utilizing single compounds- or therapeutic combinations- blocking multiple TKRs might constitute a novel improved MPNST treatment approach.
Acknowledgments
Kim Vu is thanked for aid in figure preparation. This manuscript was supported in part by a NIH/NCI RO1CA138345 (to DL) and 5T32CA009599-21 (to KT and KL) grants, an NFCR – Hope Fund Seed Grant (to DL), an Amschwand Foundation Seed Grant (to DL and KT) and a Deutsche Forschungs Gemeinschaft (to MG).
References
- 1.Zou C, Smith KD, Liu J, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249:1014–1022. doi: 10.1097/SLA.0b013e3181a77e9a. [DOI] [PubMed] [Google Scholar]
- 2.Zou CY, Smith KD, Zhu QS, et al. Dual targeting of AKT and mammalian target of rapamycin: A potential therapeutic approach for malignant peripheral nerve sheath tumor. Mol Cancer Ther. 2009;8:1157–1168. doi: 10.1158/1535-7163.MCT-08-1008. [DOI] [PubMed] [Google Scholar]
- 3.Traxler P. Tyrosine kinases as targets in cancer therapy - successes and failures. Expert Opin Ther Targets. 2003;7:215–234. doi: 10.1517/14728222.7.2.215. [DOI] [PubMed] [Google Scholar]
- 4.Katz D, Lazar A, Lev D. Malignant peripheral nerve sheath tumour (MPNST): the clinical implications of cellular signalling pathways. Expert Rev Mol Med. 2009;11:e30. doi: 10.1017/S1462399409001227. [DOI] [PubMed] [Google Scholar]
- 5.Holtkamp N, Malzer E, Zietsch J, et al. EGFR and erbB2 in malignant peripheral nerve sheath tumors and implications for targeted therapy. Neuro Oncol. 2008;10:946–957. doi: 10.1215/15228517-2008-053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellinghoff IK, Sawyers CL. The emergence of resistance to targeted cancer therapeutics. Pharmacogenomics. 2002;3:603–623. doi: 10.1517/14622416.3.5.603. [DOI] [PubMed] [Google Scholar]
- 7.Albritton KH, Rankin C, Coffin CM, et al. Phase II study of erlotinib in metastatic or unresectable malignant peripheral nerve sheath tumors (MPNST). Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings; 2006. p. 9518. [Google Scholar]
- 8.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]