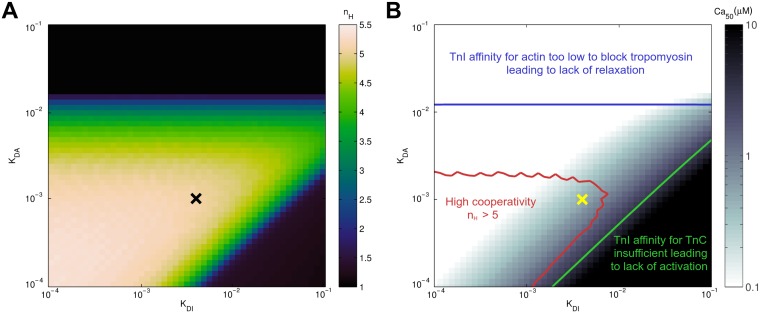
Fig 3. Influence of TnI affinity for actin and TnC on muscle cooperativity.
Panel A shows cooperativity plotted as a function of the dissociation constant of TnI for actin (K DA) and the dissociation constant of TnI for TnC⋅Ca2+ (K DI). There is a relatively large triangular region in parameter space in which cooperativity is high, with a slight tendency for higher cooperativity at very low K DA, K DI reflecting more extreme competitive binding of TnI. Panel B shows calcium sensitivity, which follows a smooth gradient The yellow ‘X’ indicates our choice of parameters, and the red contours indicate the regions within which n H ≥ 5. At high K DA, affinity for actin is insufficient to block tropomyosin effectively, leading to a permanent high level of activation (indicated by the blue text and contour line for minimum force greater than 1% of maximum force at the top of the plot). When the affinity of TnI for actin is much lower than for TnC, muscle activation is decreased, (indicated by the green text and contour line for maximum force less than 95% of overall maximum force in the bottom right of the plot).