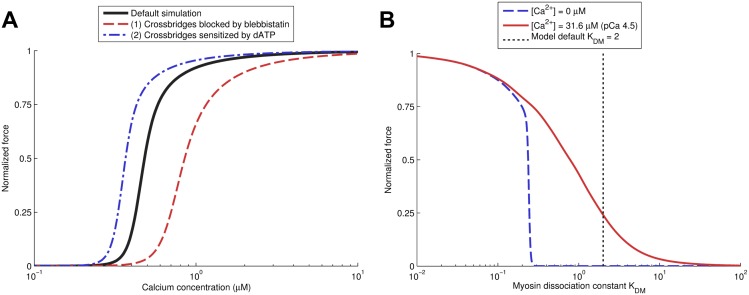
Fig 6. Force-pCa curves for the model and effects of changes in crossbridge affinity.
The effects of crossbridge inhibition by substances such as blebbistatin and sodium vanadate were simulated by changing the dissociation constant of myosin (K DM), resulting in significant changes in calcium sensitivity. Shown in panel (A) are the default, highly cooperative, force-calcium relationship of the model (n H = 5.1), along with the following virtual experiments: (1) In red: a factor 3 decrease in crossbridge affinity. This reproduces data from experiments with the cross-bridge inhibitor blebbistatin [16], showing a decrease in calcium sensitivity and a mild decrease in cooperativity (n H = 4.2). (2) In blue: a 33% increase in crossbridge affinity. This reproduces data from experiments with the cross-bridge augmenter 2-deoxy-ATP (dATP) [55], showing an increase in calcium sensitivity and a small increase in cooperativity (n H = 5.3). Panel (B) shows the K DM-dependence of force at zero Ca2+ and at pCa 4.5 [45], showing the model produces maximal force at both calcium levels for a sufficiently high myosin affinity, and a sigmoidal relationship between K DM and force. The dashed line indicates the value of K DM used in the model, which intersects the pCa 4.5 curve at approximately 0.25, the duty ratio of myosin used in the model. Thus, the maximal force generated in panel (B) for K DM → 0 is approximately 4× higher than the ‘default model’ curve in panel (A).