Figure 1. The amount of PrPSc is reduced following treatment of scrapie-infected cells with recombinant heparanase, or over-expression of heparanase.
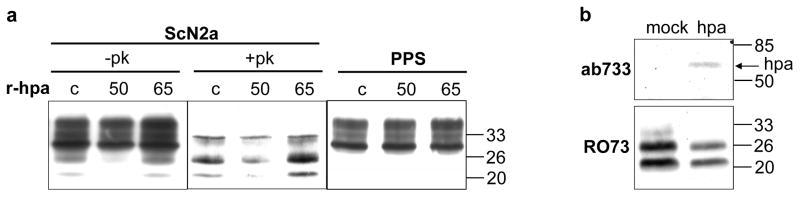
a) ScN2a-M cells (ScN2a) and pentosan polyphosphate treated N2a-M cells (PPS) growing in 6-well plates were left untreated (c) or treated (3 days, 0.1 μg/ml) with either active 50+8 kDa (50) or latent 65 kDa (65) recombinant heparanase (r-hpa). Subsequently, the cells were lysed, incubated (37 °C, 30 min) with (+PK) or without (−PK) 20 μg/ml proteinase K, and immunoblotted with mAb 3F4. b) Scrapie infected GT1-1 cells were stable transfected with human heparanase or mock-transfected. Cell lysates were subjected to SDS/PAGE and immunoblotting with anti-heparanase pAb 733 [37] (upper panel), or incubated (37°C, 30 min) with 20 μg/ml PK and immunoblotted with anti PrPSc pAb RO73 (lower panel). Notably, unlike the 3F4 mAb that recognizes the chimeric MHM2 PrPSc produced in ScN2a-M cells (Fig. 1a), the RO73 antibody detects the endogenous PrPSc produced in scrapie infected GT1-1 cells (Fig. 1b).
