Fig. 2.
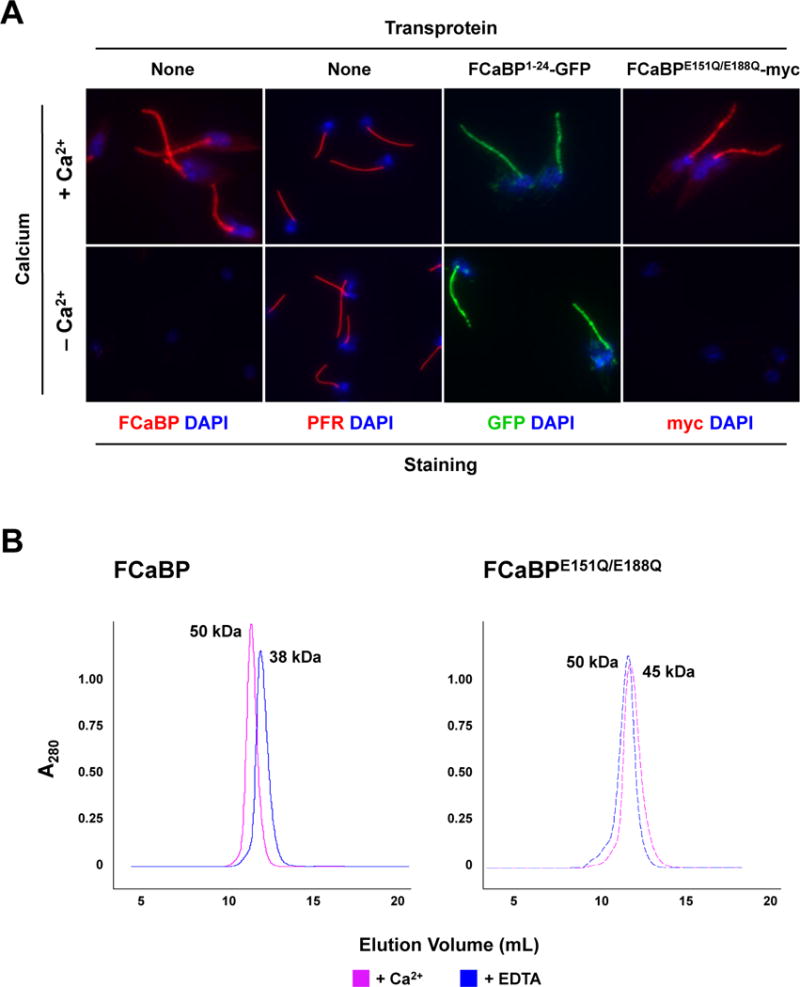
Mutation of the Ca2+ binding sites in FCaBP does not affect Ca2+-dependent flagellar membrane localization, possibly due to dimerization with endogenous wildtype FCaBP. (A) The flagellar retention of FCaBPE151Q/E188Q is Ca2+-dependent. Untransfected epimastigotes and transfectants expressing the N-terminal 24 amino acids of FCaBP fused to GFP (FCaBP1–24-GFP) or the FCaBP Ca2+-binding mutant FCaBPE151Q/E188Q-myc were permeabilized and washed with buffers containing or lacking (EDTA) Ca2+. Cells were then analyzed by direct fluorescence (GFP) or immunofluorescence microscopy with paraflagellar rod [PFR (T. cruzi PAR1)]- or FCaBP-specific antisera. DNA was stained with DAPI. Both endogenous FCaBP and FCaBPE151Q/E188Q-myc were washed out of the flagellum upon Ca2+ chelation. (B) Recombinant wildtype FCaBP and the Ca2+-binding mutant FCaBPE151Q/E188Q form dimers in a Ca2+-independent manner. Size exclusion chromatography of recombinant wildtype FCaBP and Ca2+ binding mutant FCaBPE151Q/E188Q were conducted in the presence of 2 mM CaCl2 (solid line) or 2 mM EDTA (dashed line). The absorbance at 280 nm is plotted vs elution volume. Both wildtype FCaBP and FCaBPE151Q/E188Q elute as dimers in both Ca2+-bound and Ca2+-free states. Multi-angle light scattering confirmed that FCaBP is a dimer in both Ca2+-free and Ca2+-bound states (not shown).
