Abstract
Objectives
Subjective cognitive complaints in otherwise normal aging are common but may be associated with preclinical Alzheimer Disease in some individuals. Little is known about who is mostly likely to show associations between cognitive complaints and preclinical Alzheimer pathology. We sought to 1) demonstrate associations between subjective complaints and brain amyloid-β in cognitively normal older adults; 2) to explore personality factors as potential moderators of this association.
Design
Cross-sectional observational study.
Setting
Clinical neuroimaging research center.
Participants
Community volunteer sample of 92 healthy older adults, screened for normal cognition with comprehensive neuropsychological evaluation.
Measurements
Subjective cognitive self-report measures included the Memory Functioning Questionnaire, Cognitive Failures Questionnaire, and the Subjective Cognitive Complaint Scale. Personality was measured with the NEO Five Factor Inventory. Brain amyloid-β deposition was assessed with Pittsburgh compound B (PiB)-PET imaging.
Results
One of three cognitive complaint measures, the Memory Functioning Questionnaire, was associated with global PiB retention (standardized beta =−.230, p=.046, adjusting for age, sex and depressive symptoms). Neuroticism moderated this association such that only high neuroticism individuals showed the predicted pattern of high complaint – high amyloid-β association.
Conclusions
Evidence for association between subjective cognition and brain amyloid-β deposition in healthy older adults is demonstrable but measure-specific. Neuroticism may moderate the MFQ – amyloid-β association such that it is observed in the context of higher trait neuroticism. Subjective cognitive complaints and neuroticism may reflect a common susceptibility toward psychological distress and negative affect, which are in turn risk factors for cognitive decline in aging and incident Alzheimer Disease.
Keywords: amyloid imaging, cognition, personality, subjective memory
Objective
Population studies indicate subjective cognitive complaints (SCCs) during aging are a risk factor for cognitive decline and dementia [1–3]. Neuroimaging studies have reported associations between SCCs and brain signatures of Alzheimer Disease (AD) in otherwise cognitively normal adults, including medial temporal atrophy [4, 5], reduced glucose metabolism [6, 7], and amyloid-beta (Aβ) deposition [8–10], although not all studies are positive [11]. This emerging literature has prompted interest in subjective cognitive impairment, especially in the absence of objective cognitive deficits, as a putative early neurodegenerative disease stage (i.e., pre-mild cognitive impairment; MCI) and a potential preclinical phase for intervention [12–14]. That is, the notion that some older individuals may first show insight regarding their own memory changes associated with very early AD-pathologic processes, before objectively assessed deficits, has gained recent traction [14].
However, the reasons that older adults express or endorse subjective cognitive complaints are likely complex and multifactorial. In addition to AD/brain biomarkers and objective cognitive performance, SCCs are associated with individual differences in affective variables, including mood and personality [3, 15]. In particular, two personality factors from the five factor model, high neuroticism and low conscientiousness, are consistent correlates of subjective memory / cognitive complaints [15–17]. Some authors argue that mood and personality correlates underscore the importance of psychological factors, as opposed to underlying brain dysfunction, in accounting for SCCs in aging [18, 19]. Indeed, in dementia evaluation settings, the term ‘worried well’ connotes patients who are anxious about memory changes (and perhaps anxious in general) but show no objective findings on exam; the conceptualization reflects a ruling-out of disease by the clinician [20]. Interestingly, however, a separate line of research suggests that personality traits, especially neuroticism, are themselves consistent risk factors and/or disease markers for AD and cognitive decline [21, 22]. Neuroticism is closely related to other negative affect-associated variables, such as risk for depression and vulnerability to stress, which are in turn associated with risk for AD and cognitive decline in aging [23–26]. To date, relationships among subjectively perceived cognition, personality and AD biomarkers in otherwise healthy older adults are not understood.
The aims of the present study were twofold. First, we examined associations between SCCs and Aβ deposition in cognitively normal older adults. We expected to replicate two previous studies showing association between subjective cognition and presence / degree of Aβ on imaging in CN participants [8, 9]. Secondly, we explored personality factors as potential moderating variables on associations between subjective cognition and Aβ. Regarding neuroticism, specifically, two competing hypotheses were formulated: 1) a ‘worried-well’ hypothesis would predict SCCs in the context of high neuroticism to be associated with lower risk for biomarker abnormality (i.e., low Aβ). In contrast, 2) an ‘negative-affect-risk’ hypothesis would predict SCCs in the context of high neuroticism to be associated with higher risk for biomarker abnormality (i.e., high Aβ).
Methods
Participants
Research volunteers for the present study were recruited from two ongoing PiB-PET Imaging studies at the University of Pittsburgh, one of normal aging (n=48) and amyloid and the other focused vascular-amyloid interactions in oldest-old normal aging (n=44). Cognitive classification in both parent studies was based upon a multi-domain neuropsychological assessment and review by a clinical neuropsychologist and/or multi-disciplinary consensus diagnostic procedures [27, 28]. Inclusion criteria were normal cognition and age 65 or older. Normal cognition criteria were defined as not more than 1 – 2 tests out of the multi-domain battery performed significantly below expectations given an individual’s age and educational background (i.e., scores falling more than 1 SD below age-corrected means and taking into account level of education). Exclusion criteria for both parent studies included contraindications for neuroimaging, and history of neurologic, psychiatric or other medical conditions or treatment associated with potentially significant cognitive symptoms. Psychiatric rule-out conditions included current self-reported major depression for both parent studies, and GDS > 15 for the normal aging study. Anxiety disorders were not an exclusion criterion. All participants provided written informed consent and all study procedure were approved by the Institutional Review Board of the University of Pittsburgh. Further details of recruitment and cohort characteristics are provided in Methods, Supplemental Digital Content 1.
Self-report behavioral assessments
Self-report questionnaires were administered at the time of PiB-PET imaging. Personality traits were assessed with the NEO Five-Factor Inventory (FFI-3), a 60 item questionnaire measuring the domains neuroticism, consciousness, extraversion, agreeableness, and openness-to-experience [29]. These domains reflect the five-factor model, an empirically derived structure with broad scientific consensus regarding covariation of personality traits across cultures and human development. Raw NEO-FFI scores were converted to standardized T-scores (mean 50, SD 10) according to test manual norms and procedures. Subjective cognitive complaints were assessed with the 64-item Memory Functioning Questionnaire (MFQ) [30]; the 25-item Cognitive Failures Questionnaire (CFQ) [31]; and a 24-item subjective cognitive complaints scale (SCCS) [32]. Depressive symptoms were measured with the 30-item Geriatric Depression Scale (GDS) [33].
Neuroimaging
[11C]PiB was produced as previously described [34]. Prior to PiB-PET, a 1.5 or 3T spoiled-gradient-recalled-MR was obtained for each subject for co-registration and region-of-interest (ROI) definition [34]. PET imaging was conducted using a Siemens/CTI ECAT HR + (3D mode, 15.2 cm field-of-view, 63 planes, reconstructed image resolution ~ 6 mm FWHM). The participant’s head was immobilized to minimize head motion. PiB was injected intravenously (12–15 mCi, over 20 s, specific activity ~ 1–2 Ci/μmol) and PET scanning was performed at least 50–70 min post injection. Analysis of the PiB PET data utilized standardized uptake value ratio (SUVR) (determined 50–70 min post-injection). To calculate SUVR, SUV was first determined by normalizing regional tissue radioactivity concentration to injected dose and body mass, and then each regional SUV was divided by the cerebellar reference SUV that was representative of free and nonspecific radiotracer retention.
PiB retention was quantified with a global SUVR score, the average of five cortical regions (precuneus, anterior cingulate, frontal, parietal, lateral temporal) and the striatum.
White matter hyperintensities (WMH) were included as a measure of sub-clinical cerebrovascular disease burden. WMH volume was obtained from T2-weighted FLAIR images, as previously described [35]. FLAIR images were acquired in the axial plane: TR=9160 ms; TE=90 ms; TI=2500 ms; FA=150 deg; FOV= 256*212 mm; slice thickness=3 mm; matrix size=256*212; number of slices=48 slices; and voxel size= 1 mm*1 mm*3 mm. The WMH quantification was done using a fuzzy connected algorithm [35]. The total WMH volume was normalized for brain volume.
Analysis
Primary analyses evaluated subjective cognition – Aβ associations. As a first step, we computed bivariate Pearson correlations among behavioral self-report measures, global PiB retention and key demographic/ clinical variables to guide inclusion of covariates in subsequent linear regression models. We then evaluated subjective cognition - Aβ associations with linear regression models, in which each subjective cognitive complaint questionnaire was modeled independently from the others, adjusting for age, sex, and depressive symptoms with global PiB retention as the outcome (3 subjective cognition total score measures plus 4 MFQ factor scores = 7 models). We did not include APOE*4 allele carrier status in the primary analyses because of the small number in the sample (n=14), and also because of the high degree of overlap between APOE*4 and Aβ [36]. However, secondary analyses were conducted including APOE*4 in relevant models and reported for comparison.
All correlation p-values evaluated and reported are two-tailed.
To evaluate personality factors as potential moderators of subjective cognition – Aβ associations, we created dichotomous groupings by median splits on subjective cognitive complaint measures (i.e., low vs. high subjective complaints) as well as personality measures (e.g., low vs. high neuroticism). These dichotomized variables were entered as main effects in ANOVA models, and each model included a two-way SCC × personality interaction term (the 3 subjective cognition total score measures × 5 personality measure = 15 ANOVA interaction models), with global PiB SUVR as the outcome, adjusting for age, sex and depression score. Because of the multitude of independent models uncorrected (p < .05) for multiple comparisons, these interaction models were considered exploratory.
Analyses were conducted using SPSS v. 20 and SAS 9.4.
Results
Table 1 presents descriptive characteristics, mean behavioral self-report scores and global PiB SUVR for the 92 participants. Several self-report measures had limited missing data due to date collection lags and incomplete assessments, with the maximum missing on the SCCS (n=10). Of note, the sample was somewhat older than typical neuroimaging studies of aging, with a mean age of 81 and IQR of 74 to 87 years. Mean personality factor scores were comparable to other published reports of older adults without dementia [37]. Mean subjective cognitive complaint scores were also comparable to published older adult community samples for the three subjective cognition measures (MFQ [38]; CFQ [39]; SCCS [32]).
Table 1.
Descriptive characteristics, global PiB retention, and behavioral self-report measures
| Demographic and clinical variables | |
| Age, mean (SD), y | 81.2 (8.4) |
| Education, mean (SD), y | 15.4 (2.8) |
| Male sex, n (%) | 47 (51.1 %) |
| Non-white race, n (%) | 9 (9.8 %) |
| APOE*4 carrier, n (%) | 14/80 (15.2 %) |
| GDS, mean (SD) | 3.6 (3.7) |
|
| |
| No. total medications, mean (SD) | 7.5 (3.4) |
|
| |
| Psychotropic medication use, n (%) | 12 (13.5 %) |
|
| |
| Objective cognition measures | |
|
| |
| MMSE, mean (SD) | 28.7 (1.3) |
|
| |
| Modified 24-point R-O figure delayed recall, mean (SD) | 17.4 (3.2) |
|
| |
| Trail Making Test B, s, mean (SD) | 91.9 (40.6) |
|
| |
| Animal fluency, no. in 60 s, mean (SD) | 19.4 (4.8) |
|
| |
| Digit Symbol, mean (SD) | 46.2 (12.5) |
|
| |
| Global SUV-R PiB retention | 1.77 (0.44) |
|
| |
| % WMH of total brain volume, mean (SD) | 0.645 (0.704) |
|
| |
| Subjective cognition measures | |
| MFQ (measure range 64–448, lower is worse) | 299.7 (38.1) |
| CFQ (measure range 0 – 100, higher is worse) | 34.5 (9.5) |
| SCCS (measure range 0 – 24, higher is worse) | 4.1 (3.4) |
|
| |
| Personality measures | |
| Neuroticism, T score | 41.4 (7.5) |
| Extraversion, T score | 51.5 (9.3) |
| Openness to Experience, T score | 53.1 (7.9) |
| Agreeableness, T score | 55.9 (7.7) |
| Conscientiousness, T score | 43.1 (7.5) |
Abbreviations. GDS = Geriatric Depression Scale; R-O = Rey-Osterrieth; SUV-R= standardized uptake value ratio; PiB= Pittsburgh compound B; WMH=white matter hyperintensities; MFQ = Memory Functioning Questionnaire; CFQ = Cognitive Failures Questionnaire; SCCS = Subjective Cognitive Complaints Scale.
Note. T-score mean = 50; SD = 10.
White matter hyperintensities were available in a subset of n=66 participants, due to changes in data pre-processing and technical issues (e.g., motion artifact).
Bivariate correlations among key variables
We examined zero-order correlations among self-report measures, age, education and global PiB SUVR (see Table, Supplemental Digital Content 2). Age was not a consistent correlate of self-report measures, but it was positively correlated with CFQ complaint score [r (86) =. 33, p <.05] and global PiB SUVR [r (90) = .34, p < .05]. Higher PiB SUVR was significantly correlated with lower subjective cognition on two SCC measures [CFQ r (86) = .23, p <.05; and MFQ r (82) = −.22, p<.05]. The three subjective cognition measures (MFQ, CFQ and SCCS) were significantly inter-correlated (r’s .30 to .57). Depressive symptoms (GDS) were significantly correlated with almost all subjective cognition and personality variables (r’s .22 to .56). Education was not significantly correlated with any variable of interest.
Subjective cognition – Aβ associations
Regression models were run to investigate whether subjective cognitive complaints predicted global PiB SUVR, adjusting for age, sex and depressive symptoms. Worse subjective cognition on the MFQ (total score) was associated with higher global PiB retention, with the full model results summarized in Table 2a. Neither the CFQ nor the SCCS were associated with PiB retention (see Table, Supplemental Digital Content 3, summarizing regression coefficients from adjusted models for SCC scales). Among the MFQ factor sub-scales, the General Frequency of Forgetting factor was associated with PiB retention (Table 2b).
Table 2a.
Regression model predicting global PiB retention from MFQ total score and covariates
| Unstandardized Coefficients | Standardized Coefficients | t | p | ||
|---|---|---|---|---|---|
|
| |||||
| B | Std. Error | Beta | |||
|
| |||||
| (Constant) | 1.233 | .566 | 2.178 | .032 | |
| Age | .016 | .006 | .323 | 2.825 | .006 |
| Sex | −.038 | .101 | −.045 | −.379 | .706 |
| GDS | −.002 | .012 | −.019 | −.175 | .861 |
| MFQ total score | −.003 | .001 | −.230 | −2.029 | .046 |
Model R2 = .145
Model F (4,77) = 3.264, p =.016
Table 2b.
Regression model predicting global PiB retention from MFQ ‘General Frequency of Forgetting’ factor and covariates
| Unstandardized Coefficients | Standardized Coefficients | t | p | ||
|---|---|---|---|---|---|
|
| |||||
| B | Std. Error | Beta | |||
|
| |||||
| (Constant) | 1.479 | .591 | 2.503 | .014 | |
| Age | .014 | .006 | .277 | 2.462 | .016 |
| Sex | −.047 | .100 | −.056 | −.476 | .636 |
| GDS | −.004 | .012 | −.032 | −.297 | .768 |
| MFQ General Frequency of Forgetting factor | −.005 | .002 | −.278 | −2.425 | .018 |
Model R2 = .163
Model F (4, 77) = 3.754, p =.008
Note. GDS = Geriatric Depression Scale; MFQ = Memory Functioning Questionnaire.
Subjective cognition, WMH, and number of medications
There were no significant correlations between WMH and any of the key study measures of subjective cognition, personality and Aβ deposition. Similarly, there were no significant correlations between current number of medications, subjective cognition, personality, or Aβ deposition.
Role of APOE*4 allele
Being a carrier of at least one APOE*4 allele was associated with higher global PiB retention[ Mann-Whitney U= 628 (80), p=.036] but was not associated with any of the subjective cognition measures. Of the five personality factors, APOE*4 carriers were lower on agreeableness [F(1,78)=12.00, p=.001] and conscientiousness [F(1,78)=4.63, p=.03]. Including APOE*4 carrier status in the subjective-cognition – Aβ association models did not significantly change results.
Exploratory analyses of personality as mediator
Results from the ANOVA models evaluating potential subjective cognitive complaints × personality interactions indicated one significant interaction term in the MFQ × neuroticism model (Table 3). In that model, age was the only significant main effect (Table 3). Figure 1 illustrates the nature of the MFQ × neuroticism interaction: the expected association between cognitive complaints (scaled for the figure such that higher scores correspond to more symptoms, consistent with neuroticism) and global PiB retention is apparent among the high-neuroticism group.
Table 3.
ANOVA model including MFQ × N interaction term, predicting global PiB retention
| Source | Type III Sum of Squares | df | Mean Square | F | p |
|---|---|---|---|---|---|
| Corrected Model | 2.602a | 6 | .434 | 2.694 | .020 |
| Intercept | .294 | 1 | .294 | 1.824 | .181 |
| Age | 1.014 | 1 | 1.014 | 6.302 | .014 |
| Sex | .000 | 1 | .000 | .002 | .966 |
| GDS | .051 | 1 | .051 | .318 | .574 |
| MFQ | .315 | 1 | .315 | 1.959 | .166 |
| Neuroticism | .117 | 1 | .117 | .730 | .396 |
| MFQ × Neuroticism | .768 | 1 | .768 | 4.770 | .032 |
| Error | 12.072 | 75 | .161 | ||
| Total | 263.223 | 82 | |||
| Corrected Total | 14.673 | 81 |
R2 = .177
Notes. GDS = Geriatric Depression Scale; MFQ = Memory Functioning Questionnaire (total score); N = Neuroticism
Figure 1.
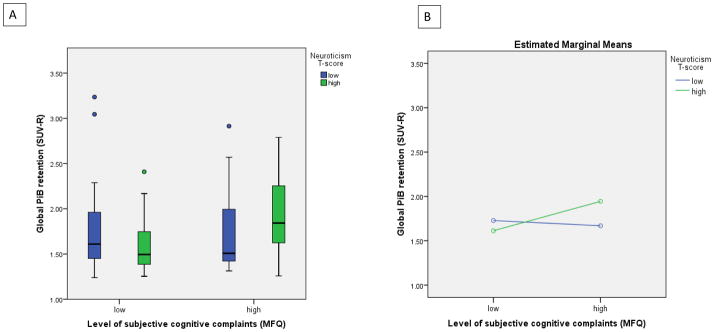
Interaction between neuroticism and subjective cognitive complaints on global PiB retention.
A). Distributions by box-plot of global PiB retention by subjective cognitive complaints and neuroticism groups (low vs high by median split on each measure). Subjective cognition was measured by the MFQ, which was reverse-scaled for this figure only to reflect low vs high level of complaints (for consistency with neuroticism scaling). B) Estimated means of global PiB SUVR from the interaction model in Table 3 (adjusted for age, sex and GDS score), showing higher PiB retention associated with poorer subjective cognition only among participants with higher neuroticism scores.
Associations between subjective and objective cognition
Among bivariate correlations between the neuropsychological measures reported in Table 1 and subjective cognition scales: better MFQ ratings were associated with better Digit Symbol performance [r (81) =.24, p< .05]; better CFQ ratings were associated with better MMSE [r (85)= −.24, p<.05], Trails B [r (85) =.21, p<.05] and Digit Symbol [r (85) = −.36, p < .01] performance; and lower complaints on the SCCS were associated with better MMSE [r (79) = −.31, p<.01] and animal fluency [r (79) = −.25, p<.05] performance. (See Table, Supplementary Digital Content 4, for complete correlation matrix).
Conclusions
The goals of this study were twofold. The first goal was to replicate recent findings that subjective cognition was associated with Aβ imaging in cognitively normal older adults. We observed this association for one of three different questionnaire measures of SCCs, while controlling for demographic variables and concurrent depressive symptoms. Thus, evidence to date for subjective cognition – amyloid associations in healthy older individuals is demonstrable but limited, i.e., not observed consistently across multiple SCC measures. In the Perrotin et al. study [8], as well, only one of 10 metacognition ratings (“general memory compared to others of the same age”) significantly differed between PiB-positive (n=11) and PiB-negative (n=28) in a smaller sample of normal volunteers. In the Amariglio et al. study [9], a subjective memory complaint composite score was generated to evaluate a memory-focused hypothesis; but of the 11 subjective cognition sub-scales reported, 3 were associated with Aβ deposition in a larger cognitively normal sample (n=131). It may be that the overall effect is small and variable among measures and samples in a non-reliable manner. Alternatively, there may be specific self-report scales that are reliably more likely to show associations with Aβ deposition / AD biomarkers, whether for psychometric- or item content-related reasons, or both. It is noteworthy that the MFQ factor General Frequency of Forgetting factor, and not the other three MFQ factors, showed a significant association in our study, as well as in the Amariglio study. This same MFQ factor pattern of association was also reported in a third imaging study [40] using [F-18] FDDNP, a less specific PET tracer reflecting Aβ as well as tau neurofibrillary tangle load in cognitively normal and MCI participants combined. We suggest the MFQ General Frequency of Forgetting factor, a 33-item sub-scale reflecting perceived frequency of memory failures in a variety of specific situations (e.g., names, faces, appointments, etc.), may be a useful candidate measure for further study in relation to preclinical AD biomarkers. Of note, none of the subjective cognition measures were related to WMH or to number of medications, as a proxy for overall health.
The second study goal was to explore five factor model personality measures as potential moderators of SCC – Aβ associations. Two factors, neuroticism and conscientiousness, are consistent correlates in the literature both of SCCs and of risk for AD / dementia / cognitive decline. Of the two, neuroticism is the mostly widely reported correlate. We observed a significant interaction between neuroticism and subjective cognition (MFQ only), such that subjective memory in the context of higher neuroticism showed the predicted association with global Aβ deposition. Individuals with poor subjective memory and high neuroticism had highest mean Aβ on imaging. No other personality factors were significant moderators.
The observed interaction is consistent with an ‘negative-affect-risk’ model of how neuroticism may relate to markers of AD risk, including subjective memory complaints. That is, both poorer cognitive self-appraisal and greater tendency toward psychological distress reflect facets of negative affect, which in turn may be generally related to dementia risk [21, 22, 25, 26, 41, 42]. Possible mechanisms include links between chronic stress and CNS alterations along the limbic (particularly hippocampal) – hypothalamic –pituitary - adrenal axis [43, 44]; risk for cardiovascular disease [45], in turn a significant risk factor for dementia; and neuroticism-associated brain structure variation in normal aging, including smaller global grey matter and regional frontal volumes [46]. As well, a direct association between neuroticism and spread of tangle pathology in limbic and neocortical regions on autopsy has been reported[47]. Finally, of note, a recent study similarly reported a moderating effect of neuroticism on the association between APOE*4 allele and both cognitive decline and incident AD[48].
In contrast to the ‘negative-affect-risk’ model, we described an alternative ‘worried well’ model which reflects the clinical notion that worry in otherwise high functioning older memory complainers is a good prognostic sign, and signals lower risk for ‘true’ (i.e., neurologic) disease. This model was not supported by our data. Our findings may also be generally consistent with evidence that cognitive complaints associated with “worry” (compared to cognitive complaints without significant worry) is more predictive of incident AD over three years among older primary care patients [1]. In the present study, however, we did not address specific worry about everyday memory functioning. We also note that the constructs ‘worry’ and ‘neuroticism’ have been shown to be distinct from each other, although correlated, in younger populations [49]. In the present study, as operationalized by scores on trait neuroticism, we observed that the tendency to toward higher emotional instability was a higher risk-state for poorer subjective cognition being associated with AD pathology. This interaction is also consistent with the notion that individuals high on neuroticism may be more likely to be perceptive of, or sensitive to, somatic symptoms in general [50] and/or to subtle changes in everyday cognitive functioning over time, specifically.
There are several limitations of the present study to note. First, clinical anxiety disorders were not assessed. While we expect the rate to be relatively low in this selected and generally healthy volunteer sample, analyses were not controlled for such conditions and we do not know the potential role they may have played. Second, the relatively older mean age of the sample (81 years) may constrain generalizability to other younger-elderly populations. An important consequence of older age distribution is the high degree of Aβ deposition [36]. In absence of frank clinical deficits (by selection) in participants surviving and thriving into the 9th decade of life, any effect of highly prevalent Aβ deposition at this age may be different, presumably attenuated, compared to a younger age [51, 52]. Future studies with larger samples are needed to compare age-specific effects. We note that the three subjective cognition measures were associated with several objective cognitive measures in the expected directions, supporting their validity in this sample. Regarding Five-Factor personality measurements in the oldest-old, these have been investigated and validated previously [53, 54]. Longitudinal studies of aging over 60 on personality traits indicate mean-level changes on Five Factor traits, including mean decrease in neuroticism, but rank-order consistency [55, 56]. This latter point supports validity of these measure in the present study.
A major limitation to note is the large number of secondary and exploratory significance tests conducted without adjustment for multiple comparisons, with consequently an increased risk of Type I error. The subjective cognition – personality interaction models were considered exploratory and the significant finding with neuroticism warrants independent replication.
In sum, we observed an association between the Memory Functioning Questionnaire ratings and global Aβ deposition in cognitively normal older neuroimaging study volunteers. We observed suggestive evidence that trait neuroticism moderated this association such that it was stronger among high-compared to low-neuroticism individuals. These findings are relevant to an evolving research interest in subjective cognitive decline as a potentially meaningful pre-MCI stage. To date, clinicians and researchers lack informative data addressing multiple etiologies of subjective cognitive complaints and the complexities of cognitive self-appraisal in aging. Individual differences in personality and affective variables may serve to better define the phenotype of subjective cognitive decline associated with an underlying neurodegenerative process.
Supplementary Material
Supplemental Digital Content 2. Table. Bivariate correlations among behavioral self-report measures, demographic variables, and global PiB SUVR
Supplemental Digital Content 3. Table. Summary of regression coefficients from adjusted * models for subjective cognitive complaint scales predicting global PiB retention (covariates not shown).
Supplemental Digital Content 4. Table. Bivariate correlations among measures of subjective and objective cognition
Acknowledgments
This study was funded by grants from the NIH-NIA: K23 AG038479; P01 AG025204, R37 AG025516; P50 AG005133.
Footnotes
Preliminary results from this study were presented at the Alzheimer’s Association International Conference in Boston, July 17, 2013.
Financial disclosures: Drs. Klunk and Mathis are co-inventors of PiB, a technology described in this manuscript. As such, they have a financial interest in the license agreement, which GE Healthcare holds with the University of Pittsburgh. GE Healthcare provided no grant support for this study and had no role in the design or interpretation of results or preparation of this manuscript Dr. Lopez has consulted for Lilly, Baxter, Lundbeck & Grifols.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jessen F, Wiese B, Bachmann C, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67(4):414–22. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 2.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15(11):983–91. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Reid LM, Maclullich AMJ. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord. 2006;22(5–6):471–85. doi: 10.1159/000096295. [DOI] [PubMed] [Google Scholar]
- 4.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834–42. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart R, Godin O, Crivello F, et al. Longitudinal neuroimaging correlates of subjective memory impairment: 4-year prospective community study. The British Journal of Psychiatry. 2011;198(3):199–205. doi: 10.1192/bjp.bp.110.078683. [DOI] [PubMed] [Google Scholar]
- 6.Mosconi L, De Santi S, Brys M, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry. 2008;63(6):609–18. doi: 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheef L, Spottke A, Daerr M, et al. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79(13):1332–1339. doi: 10.1212/WNL.0b013e31826c1a8d. [DOI] [PubMed] [Google Scholar]
- 8.Perrotin A, Mormino EC, Madison CM, et al. Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012;69(2):223. doi: 10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amariglio RE, Becker JA, Carmasin J, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012 doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mielke MM, Wiste HJ, Weigand SD, et al. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology. 2012;79(15):1570–1577. doi: 10.1212/WNL.0b013e31826e2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckley R, Saling M, Ames D, et al. Factors affecting subjective memory complaints in the AIBL aging study: biomarkers, memory, affect, and age. Int Psychogeriatr. 2013;25(08):1307–1315. doi: 10.1017/S1041610213000665. [DOI] [PubMed] [Google Scholar]
- 12.Reisberg B, Shulman MB, Torossian C, et al. Which comes first: Subjective cognitive impairment (SCI) or cognitive change? and is SCI a frequently occurring stage in the evolution of Alzheimer-associated cognitive change? Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2010;6(4):S177–S177. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia. 7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia. 2014 doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearman A, Storandt M. Predictors of subjective memory in older adults. Journals of Gerontology Series B-Psychological Sciences & Social Sciences. 2004;59(1):P4–6. doi: 10.1093/geronb/59.1.p4. [DOI] [PubMed] [Google Scholar]
- 16.Kliegel M, Zimprich D, Eschen A. What do subjective cognitive complaints in persons with aging-associated cognitive decline reflect? Int. Psychogeriatr. 2005;17(03):499–512. doi: 10.1017/s1041610205001638. [DOI] [PubMed] [Google Scholar]
- 17.Slavin MJ, Brodaty H, Kochan NA, et al. Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. The American Journal of Geriatric Psychiatry. 2010;18(8):701–710. doi: 10.1097/jgp.0b013e3181df49fb. [DOI] [PubMed] [Google Scholar]
- 18.Balash Y, Mordechovich M, Shabtai H, et al. Subjective memory complaints in elders: depression, anxiety, or cognitive decline? Acta Neurol. Scand. 2013;127(5):344–350. doi: 10.1111/ane.12038. [DOI] [PubMed] [Google Scholar]
- 19.Jungwirth S, Fischer P, Weissgram S, et al. Subjective memory complaints and objective memory impairment in the Vienna-Transdanube aging community. J Am Geriatr Soc. 2004;52(2):263–8. doi: 10.1111/j.1532-5415.2004.52066.x. [DOI] [PubMed] [Google Scholar]
- 20.Knopman DS. Subjective cognitive impairment Fickle but fateful. Neurology. 2012;79(13):1308–1309. doi: 10.1212/WNL.0b013e31826c1bd1. [DOI] [PubMed] [Google Scholar]
- 21.Wilson RS, Evans DA, Bienias JL, et al. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology. 2003;61(11):1479–85. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- 22.Terracciano A, Sutin AR, An Y, et al. Personality and risk of Alzheimer’s disease: New data and meta-analysis. Alzheimer’s & Dementia. 2013;(0) doi: 10.1016/j.jalz.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothman SM, Mattson MP. Adverse stress, hippocampal networks, and Alzheimer’s disease. Neuromolecular medicine. 2010;12(1):56–70. doi: 10.1007/s12017-009-8107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peavy GM, Jacobson MW, Salmon DP, et al. The influence of chronic stress on dementia-related diagnostic change in older adults. Alzheimer Dis Assoc Disord. 2012;26(3):260. doi: 10.1097/WAD.0b013e3182389a9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diniz BS, Butters MA, Albert SM, et al. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. The British Journal of Psychiatry. 2013;202(5):329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansson L, Guo X, Duberstein PR, et al. Midlife personality and risk of Alzheimer disease and distress A 38-year follow-up. Neurology. 2014;83(17):1538–1544. doi: 10.1212/WNL.0000000000000907. [DOI] [PubMed] [Google Scholar]
- 27.Cohen AD, Price JC, Weissfeld LA, et al. Basal cerebral metabolism may modulate the cognitive effects of Abeta in mild cognitive impairment: an example of brain reserve. J Neurosci. 2009;29(47):14770–8. doi: 10.1523/JNEUROSCI.3669-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nebes RD, Snitz BE, Cohen AD, et al. Cognitive aging in persons with minimal amyloid-β and white matter hyperintensities. Neuropsychologia. 2013;51(11):2202–2209. doi: 10.1016/j.neuropsychologia.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCrae RR, Costa J, Paul T. Brief versions of the NEO-PI-3. Journal of Individual Differences. 2007;28(3):116–128. [Google Scholar]
- 30.Zelinski EM, Gilewski MJ, Anthony-Bergstone CR. Memory Functioning Questionnaire: concurrent validity with memory performance and self-reported memory failures. Psychol Aging. 1990;5(3):388. doi: 10.1037/0882-7974.5.3.388. [DOI] [PubMed] [Google Scholar]
- 31.Broadbent DE, Cooper PF, FitzGerald P, et al. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21(Pt 1):1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 32.Snitz BE, Yu L, Crane PK, et al. Subjective Cognitive Complaints of Older Adults at the Population Level: An Item Response Theory Analysis. Alzheimer Dis Assoc Disord. 2012;26(4):344–351. doi: 10.1097/WAD.0b013e3182420bdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 34.Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25(11):1528–47. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 35.Wu M, Rosano C, Butters M, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Research: Neuroimaging. 2006;148(2):133–142. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathis CA, Kuller LH, Klunk WE, et al. In vivo assessment of amyloid-β deposition in nondemented very elderly subjects. Ann Neurol. 2013;73(6):751–761. doi: 10.1002/ana.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chapman B, Duberstein P, Tindle HA, et al. Personality predicts cognitive function over 7 years in older persons. The American Journal of Geriatric Psychiatry. 2012;20(7):612–621. doi: 10.1097/JGP.0b013e31822cc9cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilewski MJ, Zelinski EM, Schaie KW. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. [Erratum appears in Psychol Aging 1992 Jun;7(2):298] Psychol Aging. 1990;5(4):482–90. doi: 10.1037//0882-7974.5.4.482. [DOI] [PubMed] [Google Scholar]
- 39.Weaver Cargin J, Collie A, Masters C, et al. The nature of cognitive complaints in healthy older adults with and without objective memory decline. J Clin Exp Neuropsychol. 2008;30(2):245–257. doi: 10.1080/13803390701377829. [DOI] [PubMed] [Google Scholar]
- 40.Merrill DA, Siddarth P, Saito NY, et al. Self-reported memory impairment and brain PET of amyloid and tau in middle-aged and older adults without dementia. Int Psychogeriatr. 2012;24(07):1076–1084. doi: 10.1017/S1041610212000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saczynski JS, Beiser A, Seshadri S, et al. Depressive symptoms and risk of dementia The Framingham Heart Study. Neurology. 2010;75(1):35–41. doi: 10.1212/WNL.0b013e3181e62138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pietrzak RH, Scott JC, Neumeister A, et al. Anxiety symptoms, cerebral amyloid burden and memory decline in healthy older adults without dementia: 3-year prospective cohort study. The British Journal of Psychiatry. 2014;204(5):400–401. doi: 10.1192/bjp.bp.113.134239. [DOI] [PubMed] [Google Scholar]
- 43.Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proceedings of the National Academy of Sciences. 2010;107(19):8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flier JS, Underhill LH, McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 45.Roy-Byrne PP, Davidson KW, Kessler RC, et al. Anxiety disorders and comorbid medical illness. FOCUS: The Journal of Lifelong Learning in Psychiatry. 2008;6(4):467–485. doi: 10.1016/j.genhosppsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Jackson J, Balota DA, Head D. Exploring the relationship between personality and regional brain volume in healthy aging. Neurobiol Aging. 2011;32(12):2162–2171. doi: 10.1016/j.neurobiolaging.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terracciano A, Iacono D, O’Brien RJ, et al. Personality and resilience to Alzheimer’s disease neuropathology: a prospective autopsy study. Neurobiol Aging. 2013;34(4):1045–1050. doi: 10.1016/j.neurobiolaging.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dar-Nimrod I, Chapman BP, Franks P, et al. Personality factors moderate the associations between apolipoprotein genotype and cognitive function as well as late onset Alzheimer disease. The American Journal of Geriatric Psychiatry. 2012;20(12):1026–1035. doi: 10.1097/JGP.0b013e318267016b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hale WW, III, Klimstra TA, Meeus WH. Is the generalized anxiety disorder symptom of worry just another form of neuroticism? A 5-year longitudinal study of adolescents from the general population. J Clin Psychiatry. 2010;71(7):942. doi: 10.4088/JCP.09m05506blu. [DOI] [PubMed] [Google Scholar]
- 50.Costa PT, McCrae RR. Hypochondriasis, neuroticism, and aging: When are somatic complaints unfounded? Am. Psychol. 1985;40(1):19. doi: 10.1037//0003-066x.40.1.19. [DOI] [PubMed] [Google Scholar]
- 51.Snitz BE, Weissfeld LA, Lopez OL, et al. Cognitive trajectories associated with β-amyloid deposition in the oldest-old without dementia. Neurology. 2013;80(15):1378–1384. doi: 10.1212/WNL.0b013e31828c2fc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopez OL, Klunk WE, Mathis C, et al. Amyloid, neurodegeneration, and small vessel disease as predictors of dementia in the oldest-old. Neurology. 2014;83(20):1804–1811. doi: 10.1212/WNL.0000000000000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roepke S, McAdams L, Lindamer L, et al. Personality profiles among normal aged individuals as measured by the NEO-PI-R. Aging & Mental Health. 2001;5(2):159–164. doi: 10.1080/13607860120038339. [DOI] [PubMed] [Google Scholar]
- 54.Kato K, Zweig R, Barzilai N, et al. Positive attitude towards life and emotional expression as personality phenotypes for centenarians. Aging (Albany NY) 2012;4(5):359. doi: 10.18632/aging.100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts BW, Walton KE, Viechtbauer W. Patterns of mean-level change in personality traits across the life course: a meta-analysis of longitudinal studies. Psychol Bull. 2006;132(1):1. doi: 10.1037/0033-2909.132.1.1. [DOI] [PubMed] [Google Scholar]
- 56.Roberts BW, DelVecchio WF. The rank-order consistency of personality traits from childhood to old age: a quantitative review of longitudinal studies. Psychol Bull. 2000;126(1):3. doi: 10.1037/0033-2909.126.1.3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 2. Table. Bivariate correlations among behavioral self-report measures, demographic variables, and global PiB SUVR
Supplemental Digital Content 3. Table. Summary of regression coefficients from adjusted * models for subjective cognitive complaint scales predicting global PiB retention (covariates not shown).
Supplemental Digital Content 4. Table. Bivariate correlations among measures of subjective and objective cognition


