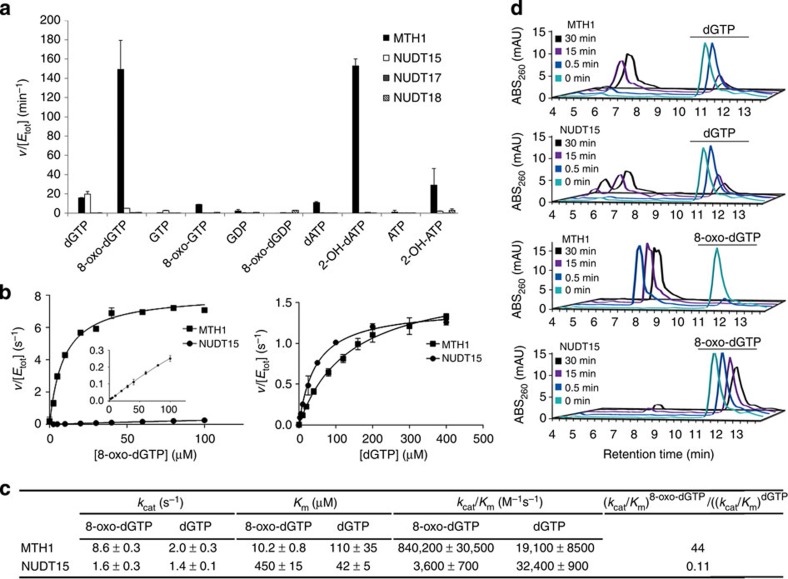
Figure 1. Comparison of NUDIX protein activity with nucleotide substrates.
(a) Nucleotide substrate (50 μM) was incubated with 5–500 nM NUDIX protein depending on enzyme. Hydrolysis was monitored by detecting phosphate generated. The depicted data are representative of two independent experiments showing the same result. Data are presented as v (hydrolysed substrate (μM) per minute) per [enzyme] (μM). (b) Saturation curves and kinetic parameters of MTH1- and NUDT15-mediated hydrolysis of 8-oxo-dGTP (left) and dGTP (right). NUDT15 (8 nM) or MTH1 (0.25 nM) was incubated with 8-oxo-dGTP at concentrations ranging from 0 to 100 μM in assay buffer, and initial rates were determined in duplicate. Inset highlights NUDT15 activity on a smaller activity scale. NUDT15 (8 nM) and MTH1 (2 nM) were incubated with dGTP in assay buffer ranging from 0 to 400 μM, and initial rates were determined in duplicate. Data are presented as v (hydrolysed substrate (μM) per second) per [enzyme] (μM), and are representative of data collected from at least two independent experiments. (c) Kinetic parameters of MTH1 and NUDT15 for dGTP and 8-oxo-dGTP hydrolysis. The Michaelis–Menten equation was applied to saturation curves using the GraphPad Prism software and kinetic parameters were calculated. Data presented are average±s.d. from two independent experiments. (d) HPLC chromatograms showing the activity of NUDT15 and MTH1 against 8-oxo-dGTP and dGTP. The depicted data are representative of three independent experiments and show that MTH1 rapidly hydrolyses 8-oxo-dGTP; however, no significant activity is observed with NUDT15. Both MTH1 and NUDT15 can hydrolyse dGTP.