Figure 4.
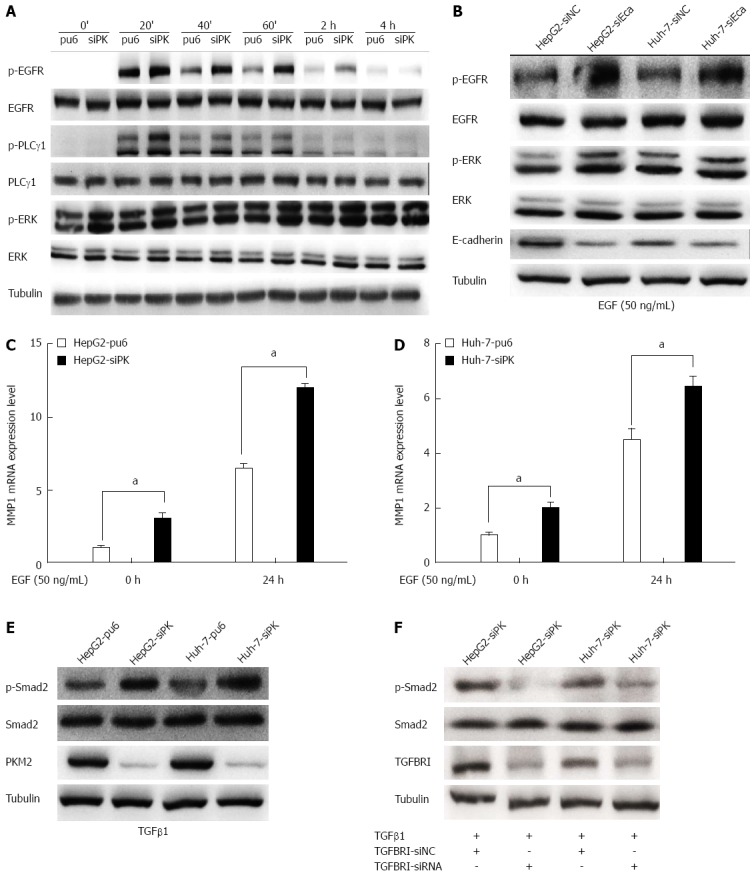
Depletion of pyruvate kinase M2 enhanced the activities of the epidermal growth factor/EGFR and transforming growth factor beta 1/TGFBR downstream signaling pathways. A: Stable HepG2 and Huh-7 cells were exposed to epidermal growth factor (EGF) (50 ng/mL) for different times. Western blots of the cell lysates are shown. The protein levels of phospho-EGFR (Tyr1068), phospho-PLCγ1 (Tyr783), and phospho-ERK1/2 (Thr202/Tyr204) are shown as indicated; B: The expression of E-cadherin was knocked down in HepG2 and Huh-7 cells by transient transfection of siRNA, and after 48 h, these cells were stimulated with EGF (50 ng/mL). The protein levels of phospho-EGFR (Tyr1068) and phospho-ERK1/2 (Thr202/Tyr204) are shown as indicated; C and D: MMP1 expression levels were analyzed by quantitative real-time PCR in HepG2 and Huh-7 stable cells. Error bars represent the mean ± SD of triplicate experiments (aP < 0.05 between groups); E: Phospho-Smad2 (Ser465/467) protein levels are shown as indicated in stable HepG2 and Huh-7 cells stimulated with TGFβ1 (20 ng/mL); F: The expression of TGFBRI was knocked down in HepG2 and Huh-7 cells by transient transfection of siRNA, and after 48 h, these cells were stimulated with transforming growth factor beta 1(TGFβ1) (20 ng/mL). The protein levels of phospho-Smad2 (Ser465/467) are shown as indicated.
