Scheme 2.
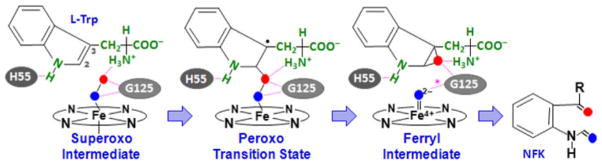
Proposed ferryl-based dioxygenase mechanism. The scheme is based on the reaction involving tryptophan dioxygenase from Xanthomonas campestris; a similar mechanism is applicable to human indoleamine 2,3-dioxygenase (hIDO), except that in hIDO H55 is replaced by S167, which is incapable of forming a hydrogen bond with the indole amine group of the substrate, G125 is replaced by A264, and the hydrogen bond indicated by the asterisk in the ferryl intermediate is absent
