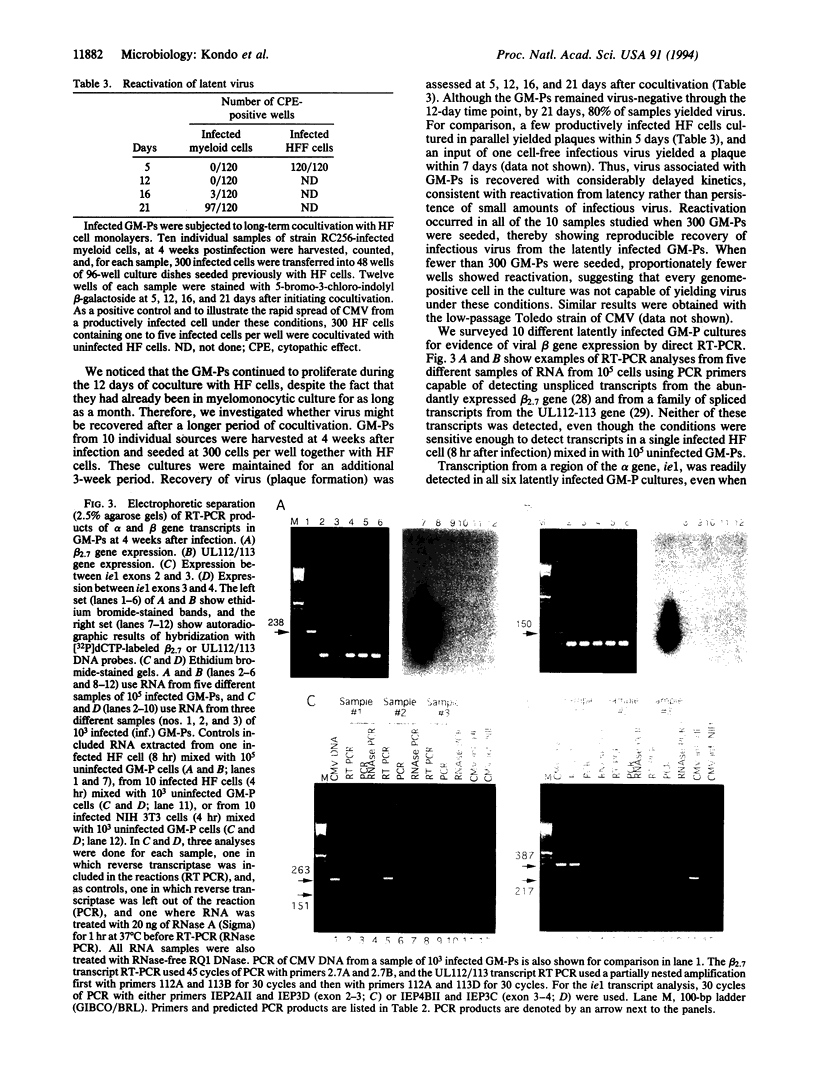
Abstract
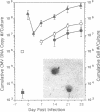
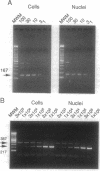
We have investigated the interaction of human cytomegalovirus (CMV) with cultured primary granulocyte-macrophage progenitors, a suspected natural site of viral latency, and have established conditions for latent infection and reactivation in this cell population. Progenitor cells from human fetal liver or bone marrow maintained a CD14+, CD15+, CD33+ cell surface phenotype during propagation in suspension culture. Exposure to human CMV did not reduce growth or alter the phenotype of these cells during a 4-week culture period. Viral replication was not detectable in these cells, although viral DNA, as measured by PCR analysis, persisted in a high proportion of cultured cells in the absence of delayed early (beta) gene expression. Viral gene expression was restricted such that only ie1 region transcripts were detected by PCR analysis of cDNA, and these transcripts were estimated to be present in no less than 2-5% of latently infected cells. Most of these transcripts remained unspliced, a result that strikingly contrasts with the splicing pattern normally seen during viral replication in permissive cells. Latent virus reactivated after prolonged, 16- to 21-day cocultivation of infected granulocyte-macrophage progenitors with permissive cells, results that support a role for the myelomonocytic cell population as a biological reservoir of latent human CMV and suggest that these cells may be the source of CMV DNA PCR-positive monocytes found in the peripheral blood of healthy carriers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apperley J. F., Dowding C., Hibbin J., Buiter J., Matutes E., Sissons P. J., Gordon M., Goldman J. M. The effect of cytomegalovirus on hemopoiesis: in vitro evidence for selective infection of marrow stromal cells. Exp Hematol. 1989 Jan;17(1):38–45. [PubMed] [Google Scholar]
- Baines P., Masters G., Booth M., Jacobs A. Enrichment of progenitor cells from human marrow. Exp Hematol. 1987 Aug;15(7):809–813. [PubMed] [Google Scholar]
- Bevan I. S., Daw R. A., Day P. J., Ala F. A., Walker M. R. Polymerase chain reaction for detection of human cytomegalovirus infection in a blood donor population. Br J Haematol. 1991 May;78(1):94–99. doi: 10.1111/j.1365-2141.1991.tb04388.x. [DOI] [PubMed] [Google Scholar]
- Braun R. W., Reiser H. C. Replication of human cytomegalovirus in human peripheral blood T cells. J Virol. 1986 Oct;60(1):29–36. doi: 10.1128/jvi.60.1.29-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenaway P. J., Wilkinson G. W. Nucleotide sequence of the most abundantly transcribed early gene of human cytomegalovirus strain AD169. Virus Res. 1987 Feb;7(1):17–31. doi: 10.1016/0168-1702(87)90055-4. [DOI] [PubMed] [Google Scholar]
- Ibanez C. E., Schrier R., Ghazal P., Wiley C., Nelson J. A. Human cytomegalovirus productively infects primary differentiated macrophages. J Virol. 1991 Dec;65(12):6581–6588. doi: 10.1128/jvi.65.12.6581-6588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K., Kondo T., Okuno T., Takahashi M., Yamanishi K. Latent human herpesvirus 6 infection of human monocytes/macrophages. J Gen Virol. 1991 Jun;72(Pt 6):1401–1408. doi: 10.1099/0022-1317-72-6-1401. [DOI] [PubMed] [Google Scholar]
- Kyoizumi S., Baum C. M., Kaneshima H., McCune J. M., Yee E. J., Namikawa R. Implantation and maintenance of functional human bone marrow in SCID-hu mice. Blood. 1992 Apr 1;79(7):1704–1711. [PubMed] [Google Scholar]
- Lathey J. L., Spector S. A. Unrestricted replication of human cytomegalovirus in hydrocortisone-treated macrophages. J Virol. 1991 Nov;65(11):6371–6375. doi: 10.1128/jvi.65.11.6371-6375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski E. S., Bonyhadi M., Salimi S., McCune J. M., Kaneshima H. Human cytomegalovirus in a SCID-hu mouse: thymic epithelial cells are prominent targets of viral replication. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):104–108. doi: 10.1073/pnas.90.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namikawa R., Weilbaecher K. N., Kaneshima H., Yee E. J., McCune J. M. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990 Oct 1;172(4):1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin S. A., Starr S. E., Friedman H. M., Gönczöl E., Weibel R. E. Protective effects of Towne cytomegalovirus vaccine against low-passage cytomegalovirus administered as a challenge. J Infect Dis. 1989 May;159(5):860–865. doi: 10.1093/infdis/159.5.860. [DOI] [PubMed] [Google Scholar]
- Porter-Jordan K., Rosenberg E. I., Keiser J. F., Gross J. D., Ross A. M., Nasim S., Garrett C. T. Nested polymerase chain reaction assay for the detection of cytomegalovirus overcomes false positives caused by contamination with fragmented DNA. J Med Virol. 1990 Feb;30(2):85–91. doi: 10.1002/jmv.1890300202. [DOI] [PubMed] [Google Scholar]
- Reiser H., Kühn J., Doerr H. W., Kirchner H., Munk K., Braun R. Human cytomegalovirus replicates in primary human bone marrow cells. J Gen Virol. 1986 Dec;67(Pt 12):2595–2604. doi: 10.1099/0022-1317-67-12-2595. [DOI] [PubMed] [Google Scholar]
- Rice G. P., Schrier R. D., Oldstone M. B. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier R. D., Nelson J. A., Oldstone M. B. Detection of human cytomegalovirus in peripheral blood lymphocytes in a natural infection. Science. 1985 Nov 29;230(4729):1048–1051. doi: 10.1126/science.2997930. [DOI] [PubMed] [Google Scholar]
- Scott D. M., Rodgers B. C., Freeke C., Buiter J., Sissons J. G. Human cytomegalovirus and monocytes: limited infection and negligible immunosuppression in normal mononuclear cells infected in vitro with mycoplasma-free virus strains. J Gen Virol. 1989 Mar;70(Pt 3):685–694. doi: 10.1099/0022-1317-70-3-685. [DOI] [PubMed] [Google Scholar]
- Simmons P., Kaushansky K., Torok-Storb B. Mechanisms of cytomegalovirus-mediated myelosuppression: perturbation of stromal cell function versus direct infection of myeloid cells. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1386–1390. doi: 10.1073/pnas.87.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sing G. K., Ruscetti F. W. Preferential suppression of myelopoiesis in normal human bone marrow cells after in vitro challenge with human cytomegalovirus. Blood. 1990 May 15;75(10):1965–1973. [PubMed] [Google Scholar]
- Spaete R. R., Mocarski E. S. Insertion and deletion mutagenesis of the human cytomegalovirus genome. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7213–7217. doi: 10.1073/pnas.84.20.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staprans S. I., Spector D. H. 2.2-kilobase class of early transcripts encoded by cell-related sequences in human cytomegalovirus strain AD169. J Virol. 1986 Feb;57(2):591–602. doi: 10.1128/jvi.57.2.591-602.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Wiedeman J., Sissons J. G., Borysiewicz L. K., Sinclair J. H. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991 Sep;72(Pt 9):2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- Taylor-Wiedeman J., Sissons P., Sinclair J. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J Virol. 1994 Mar;68(3):1597–1604. doi: 10.1128/jvi.68.3.1597-1604.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods G. L., Young A., Johnson A., Thiele G. M. Detection of cytomegalovirus by 24-well plate centrifugation assay using a monoclonal antibody to an early nuclear antigen and by conventional cell culture. J Virol Methods. 1987 Dec;18(4):207–213. doi: 10.1016/0166-0934(87)90082-6. [DOI] [PubMed] [Google Scholar]