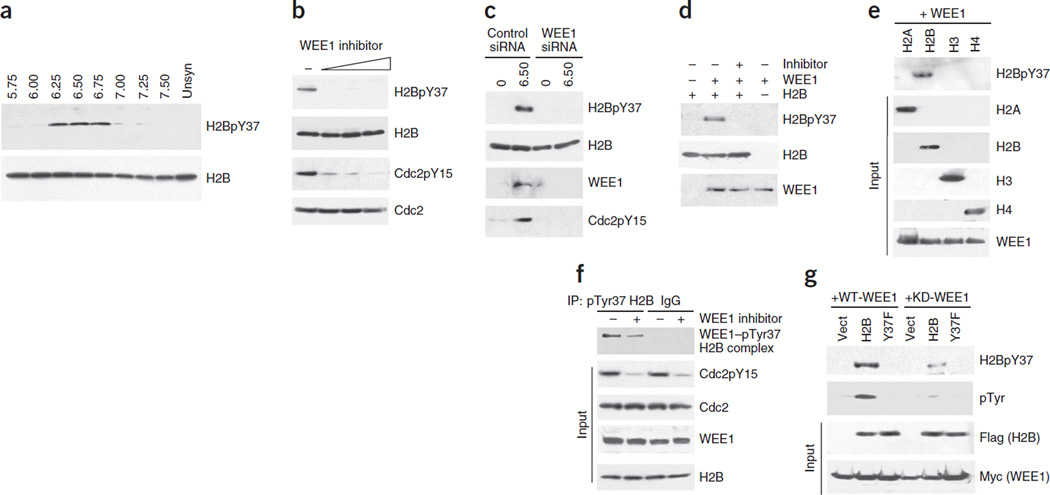
Figure 1.
WEE1 phosphorylates histone H2B at Tyr37. (a) Synchronized MEF lysates immunoprecipitated with pTyr37 H2B antibodies and immunoblotted with H2B antibody (top). Total levels of histone H2B are shown in the bottom blot. Unsyn, unsynchronized. (b) H1975 cells treated with the WEE1 inhibitor MK-1775 (0.31, 0.62 and 1.25 µM) had loss of H2B Tyr37 phosphorylation. (c) MEFs transfected with WEE1 siRNA had loss of H2B Tyr37 phosphorylation compared to cells transfected with control siRNAs. (d) In vitro kinase assay with purified proteins showing direct phosphorylation of H2B by WEE1 kinase. (e) In vitro kinase assay with purified proteins indicating that WEE1 kinase phosphorylates H2B but not other core histones. (f) Coimmunoprecipitation revealing endogenous WEE1–pTyr37 H2B complexes in MEFs. (g) MEFs coexpressing Myc-tagged WEE1 (WT-WEE1) or kinase-dead mutant WEE1 (KD-WEE1) and empty vector, Flag-tagged H2B or Y37F mutant H2B were immunoprecipitated with pTyr37 H2B antibodies and then immunoblotted with Flag antibody, revealing that WEE1 specifically phosphorylated H2B at Tyr37.