Figure 6.
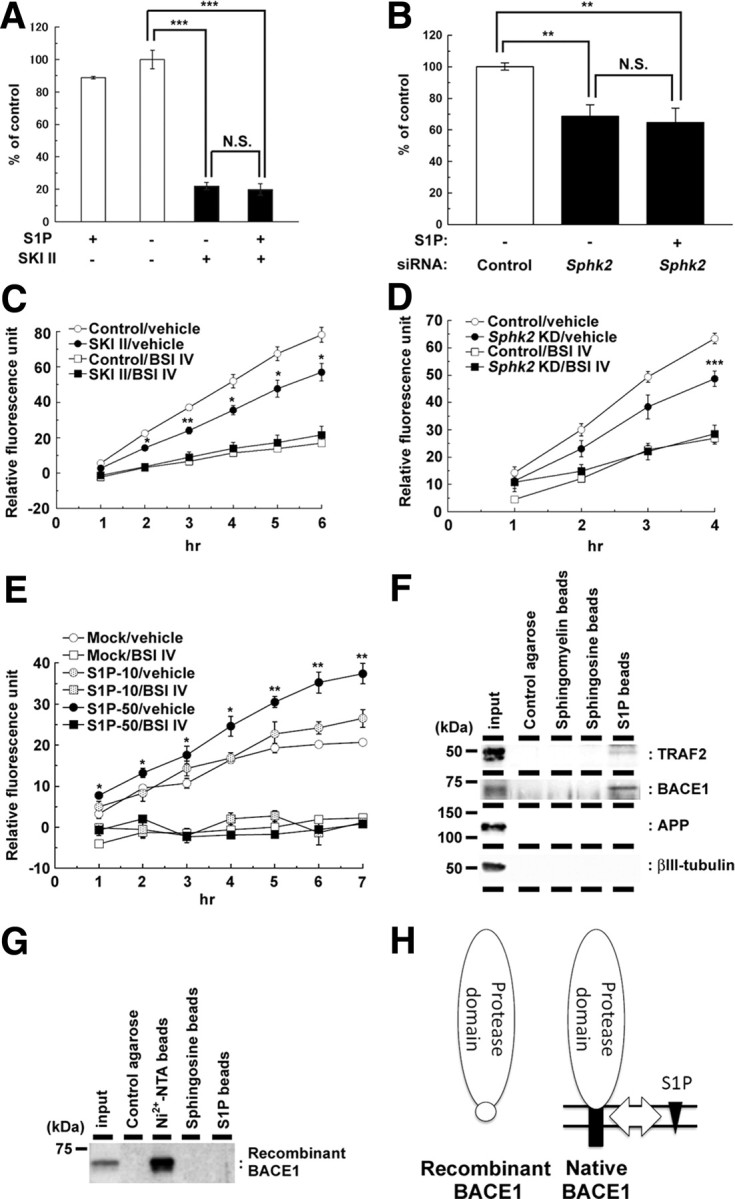
SKI II treatment decreased the β-secretase activity in cellular membrane. A, B, Effect of extracellularly added S1P (10 μm) on levels of secreted Aβ from mouse primary cortical neurons (7 d in vitro) after treatment with SKI II (1 μm) for 24 h (A) (n = 4; mean ± SEM) or from N2a cells after 48 h SphK2 knockdown (B) (n = 3; mean ± SEM; **p < 0.01, ***p < 0.001; N.S., no significant difference). Note that S1P failed to rescue the decrease in Aβ production either by SKI II or SphK2 knockdown. C, β-Secretase activity in the membrane fractions of N2a cells treated with vehicle or SKI II (1 μm) for 24 h. BACE inhibitor IV (BSI IV; 1 μm) was added to the in vitro assay (n = 3; mean ± SEM; *p < 0.05, **p < 0.01 vs control/vehicle). D, β-Secretase activity in the membrane fractions of SphK2 knockdown N2a cells. BACE inhibitor IV (BSI IV; 1 μm) was added to the in vitro assay (n = 3; mean ± SEM; ***p < 0.001 vs control/vehicle). E, Effect of S1P on β-secretase activity in the membrane fractions of mouse brain. S1P (10 or 50 μm) and BACE inhibitor IV (BSI IV; 1 μm) were added to the in vitro assay (n = 3; mean ± SEM; *p < 0.05, **p < 0.01 vs mock/vehicle). F, G, Association of BACE1 holoprotein with immobilized S1P. N2a cell lysates (F) or recombinant BACE1 with 10×His tag that lacks the transmembrane and cytoplasmic domains (G) were incubated with control agarose (no lipid), Nickel-NTA agarose, sphingomyelin, sphingosine, or S1P-coated affinity matrices (as indicated), and bound proteins were analyzed by immunoblotting. H, Schematic model of the binding of BACE1 and S1P. S1P (black triangles) interacts with the C-terminal region of BACE1 (black squares), including the transmembrane domain, but not with the extracellular protease domain (white ovals). Location of 10×His tag is indicated by a white circle.
