Figure 1.
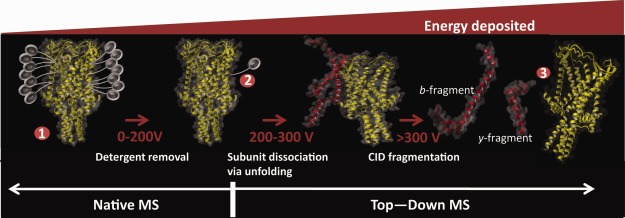
Effect of different energy regimes used in mass spectrometry of membrane proteins under native conditions when using sugar based detergents, such as the commonly used detergent DDM. The apparent protective ability of detergent micelles in the gas-phase allow noncovalent interactions to be maintained for membrane proteins at levels (up to 200 V) at which soluble protein complexes would already undergo significant dissociation. Increasing the collision energy above the threshold for membrane protein release leads to collision induced unfolding, followed by dissociation and even fragmentation, yielding sequence information. Collision energies reported are typical values for DDM, but will vary based on the protein and detergents used.
