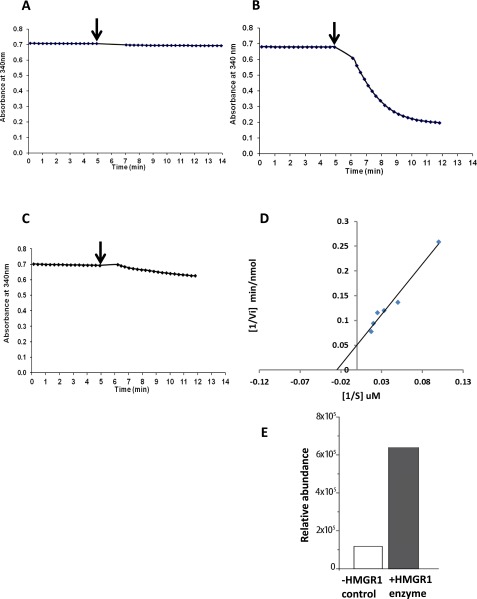
Fig. S1.
Enzymatic analysis of MtHMGR1 activity. To evaluate the catalytic activity of M. truncatula HMGR1, assays were performed by measuring the oxidation of NADPH in the presence of the substrate HMG-CoA. (A) There is no detectible change in the oxidation of NADPH in the absence of MtHMGR1. (B) However, in the presence of HMGR1, addition of HMG-CoA results in a progressive oxidation of NADPH. (C) Addition of the competitive inhibitor lovastatin (50 µM) to the reaction mixture inhibits HMGR1 activity, confirming that MtHMGR1 has HMG-CoA reductase activity. Arrows indicate the addition of HMG-CoA to initiate the reaction, and the oxidation of NADPH was determined by measuring the absorbance at 340 nm. (D) The double reciprocal Lineweaver–Burk plot was used to calculate the Vmax as 3.62 ± 0.37 µmoles/min/mg of protein and Km as 38.88 ± 4.41 µM. (E) Confirmation of the production of MVA in HMGR1 ezymatic assay as determined by ion chromatogram obtained through ultra gas chromatograph coupled to a quadrupole/orbitrap mass spectrometer. MVA production in the HMGR1 enzymatic assay was 5 times greater than the control.