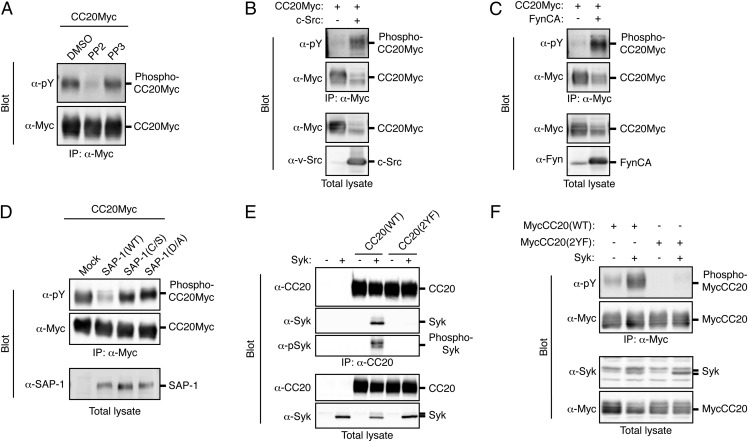
Fig. 5.
Tyrosine phosphorylation of CEACAM20 by SFKs and its association with Syk. (A) HEK293A cells expressing Myc-epitope–tagged CEACAM20 (CC20Myc) were treated with 10 μM PP2 or PP3 (or with DMSO vehicle) for 30 min, after which cell lysates were subjected to immunoprecipitation with antibodies to Myc and the resulting precipitates were subjected to immunoblot analysis with antibodies to Myc or to phosphotyrosine (α-pY). (B and C) Lysates of HEK293A cells transfected with an expression vector for CC20Myc together with either an expression vector for c-Src (B), an active mutant of Fyn [Fyn CA] (C), or the corresponding empty vector (B and C) were subjected to immunoprecipitation and immunoblot analysis as in A. Total cell lysates were also subjected to immunoblot analysis with antibodies to Myc (B and C), to v-Src (B), or to Fyn (C). (D) Lysates of HEK293A cells transfected with expression vectors for the indicated proteins were subjected to immunoprecipitation and immunoblot analysis as in A. Total cell lysates were also subjected to immunoblot analysis with antibodies to SAP-1. (E) Lysates of HEK293A cells transfected with expression vectors for the indicated proteins were subjected to immunoprecipitation with antibodies to CEACAM20 (α-CC20), and the resulting precipitates as well as the original cell lysates were subjected to immunoblot analysis with antibodies to CEACAM20, to Syk, or to phosphorylated Syk (α-pSyk). (F) Lysates of HEK293A cells transfected with expression vectors for the indicated proteins were subjected to immunoprecipitation with antibodies to Myc, and the resulting precipitates as well as the original cell lysates were subjected to immunoblot analysis with the indicated antibodies. All data are representative of three independent experiments.