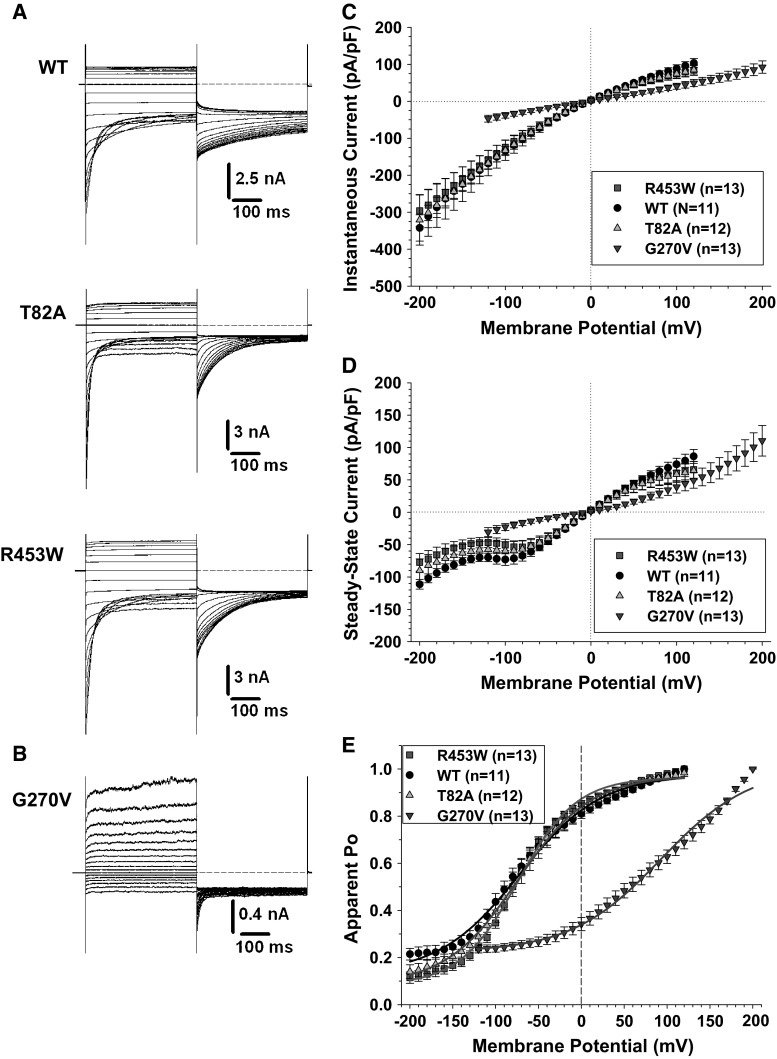
Fig. 3.
Chloride currents generated by wild-type hClC-1 channels and MC ClC-1 variants in high intracellular chloride condition. a Typical chloride currents recorded in HEK293 cells transfected with wild-type, p.T82A, or p.R453W hClC-1 variants. Cells are held at 0 mV, and 400-ms voltage pulses are applied from −200 to +120 mV in 10-mV intervals every 3 s. For clarity, only current traces obtained every 20 mV are shown. b Voltage pulses are applied from −120 to +200 mV to elicit chloride currents in HEK293 cells expressing p.G270V hClC-1 variant. c The instantaneous currents are measured at the beginning of test voltage pulses, normalized with respect to cell capacitance (pA/pF), and reported as a function of voltage. Each point is the mean ± SEM from 11 to 13 cells. Similar current density and strong inward rectification are observed for WT, p.T82A, and p.R453W channels. The relationship for p.G270V channels is linear. d Steady state currents are measured at the end of test voltage pulses and reported as mean current density ±SEM in function of voltage. Again, relationships for WT, p.T82A, and p.R453W channels are very similar, whereas current density and rectification are different for p.G270V. e The voltage dependence of activation is determined by plotting the apparent open probability (Po), calculated from tail currents measured at −105 mV, as a function of test voltage pulses. The relationships obtained from averaged data are fitted with a Boltzmann equation, and fit parameters are reported in Table 1. The activation curves for WT, p.T82A, and p.R453W are superimposed, whereas p.G270V channels displayed voltage dependence greatly shifted toward positive voltages