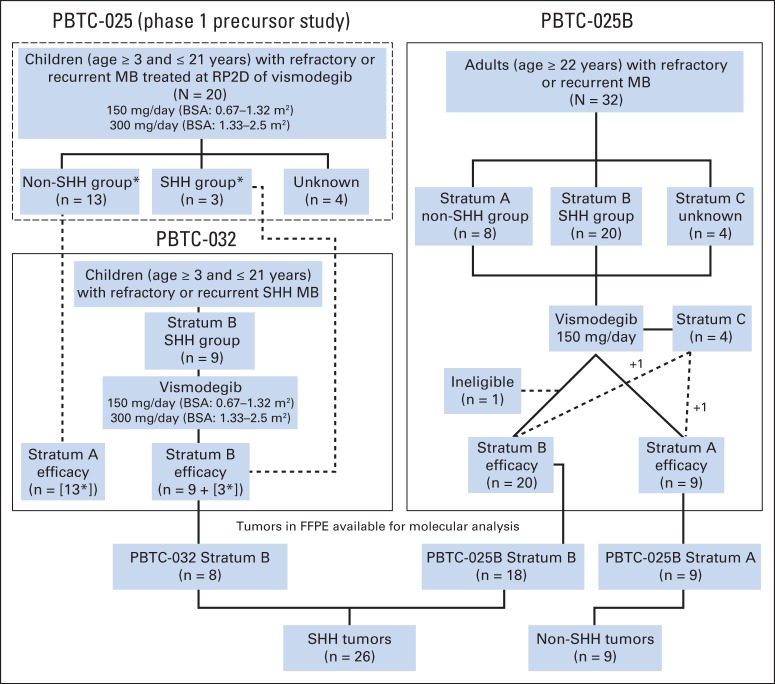
Fig 2.
Trial schematics and distribution of patients from PBTC-025 (phase I precursor study), PBTC-032 (children), and PBTC-025B (adults). In PBTC-032, strata A and C were closed to accrual before study opening, because no objective responses were seen in 13 patients with non–sonic hedgehog (SHH) medulloblastoma (MB) treated at recommended phase II dose (RP2D) of vismodegib during phase I study PBTC-025. (*) Phase I PBTC-025 patients treated at the RP2D counted toward the phase II (PBTC-032) accrual. Bottom panel shows distribution of available formalin-fixed, paraffin-embedded (FFPE) tumor samples for molecular analysis. BSA, body surface area.