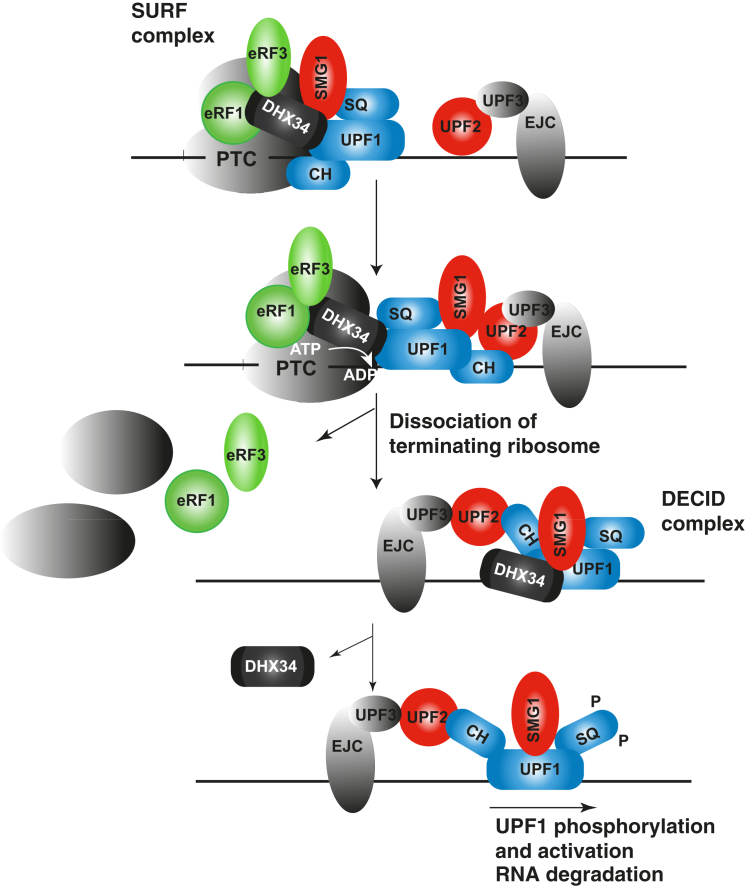
Figure 7.
Model Depicting the Role of DHX34 in NMD Activation
A translation termination event upstream of an exon junction complex leads to the recruitment of UPF1 and SMG1 by the eukaryotic translation release factors eRF1 and eRF3, forming the surveillance complex (SURF). At this stage, UPF1 activity is repressed by two distinct intramolecular interactions mediated by the N-terminal CH domain and the C-terminal SQ domain (Chakrabarti et al., 2011; Fiorini et al., 2013). During the assembly of the decay-inducing complex (DECID), the interaction of UPF1 with UPF2 induces a large conformational change in the regulatory CH domain of UPF1; however, complete activation is only achieved when repression of its SQ domain is relieved and the bound SMG1 kinase phosphorylates UPF1 on its SQ domain. This is accompanied by the displacement of the ribosome and the eRFs from the RNP complexes. This conversion is enhanced by DHX34, which associates with the SURF complex and promotes the remodeling of the SURF complex. DHX34 triggers the release of the release factors eRF1 and eRF3 in an ATP-hydrolysis-dependent manner and promotes the interaction of UPF1 with UPF2 and additional EJC proteins and induces the transition to the DECID complex that targets the RNA for decay.