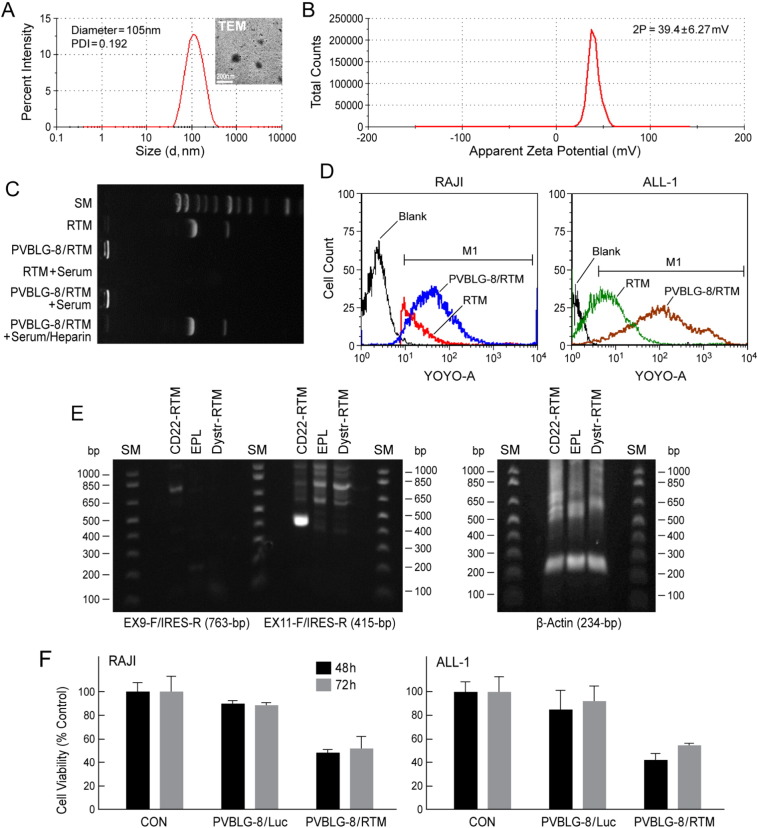
Fig. 6.
PVBLG-8-based nanoparticle (NP) formulation (NPF) of CD22-RTM.
(A) DLS analysis of PVBLG-8/RTM (weight ratio 10:1) nanoparticles, showing a particle size around 105-nm in diameter and with a narrow polydispersity index of 0.192. Insert: TEM image of PVBLG-8/RTM NP. (B) Zeta-potential result of PVBLG-8/RTM NP, showing that the NP were positively charged. (C) CD22-RTM condensation by PVBLG-8 at a PVBLG-8/CD22-RTM weight ratio of 10:1, as evaluated by a gel retardation assay, as previously reported (Zheng et al., 2014). For the CD22-RTM + serum or PVBLG-8/CD22-RTM + serum conditions, CD22-RTM or PVBLG-8/CD22-RTM was incubated with serum for 2 h at 37 °C, respectively. For PVBLG-8/CD22-RTM + serum/heparin, after incubation with serum, a 10-fold excess heparin was added to remove the CD22-RTM from the NP. (D) Flow cytometric analysis of the cellular delivery of fluorescent-labeled CD22-RTM by PVBLG-8/CD22-RTM NP. Unformulated YOYO1-CD22 RTM vs. PVBLG-8/YOYO1-CD22 RTM NP were incubated with CD22ΔE12+ RAJI (Burkitt's lymphoma/leukemia) or ALL-1 (BCR-ABL+ B-lineage ALL) cells (0.5 μg DNA/sample) for 4 h. After 2 washes with heparin-containing PBS to remove membrane-bound NP, cells were fixed with paraformaldehyde (4%, 100 μL) and analyzed for cell-associated fluorescence caused by internalized YOYO1-CD22 RTM using flow cytometry. Markedly improved CD22-RTM delivery to RAJI and ALL-1 cells was confirmed for the NP formulation by the higher cell-associated fluorescence intensity in samples incubated with PVBLG-8/YOYO1-CD22 RTM vs. unformulated YOYO1-CD22 RTM. RTM: in this figure, abbreviation for CD22-RTM. (E) RAJI cells in 1 mL culture medium were incubated for 4 h at 37 °C with PVBLG-8 (50 μg)/CD22-RTM (5 μg), PVBLG-8 (50 μg)/Dystrophin-RTM (5 μg), or PVBLG-8 (50 μg)/empty plasmid (EPL) (5 μg). Cells were washed twice and then cultured in RPMI + 10% FBS for 48 h at 37 °C/5% CO2. Total RNA was extracted from the 48 h samples and subjected to RT-PCR analysis. Depicted is an agarose gel that shows the RT-PCR evidence for successful transfection and trans-splicing, as described in the legend of Fig. 5. Also depicted is an agarose gel showing the RT-PCR amplification of β-actin mRNA (as a control for RNA integrity) with a forward primer 5′-GGACTTCGAGCAAGAGATGG-3′, and a reverse primer 5′-AGCACTGTGTTGGCGTACAG-3′. This primer set amplifies a 234-bp region at the junction between Exon 4 and Exon 5 of the human beta actin gene. (F) Anti-leukemic activity of PVBLG-8/CD22-RTM nanoparticles. Depicted are the MTT viability assay data showing the anti-leukemic effects of PVBLG-8 (50 μg)/CD22-RTM (5 μg) NP vs. control NP PVBLG-8 (50 μg)/pLuc (5 μg) on RAJI and ALL-1 cells.