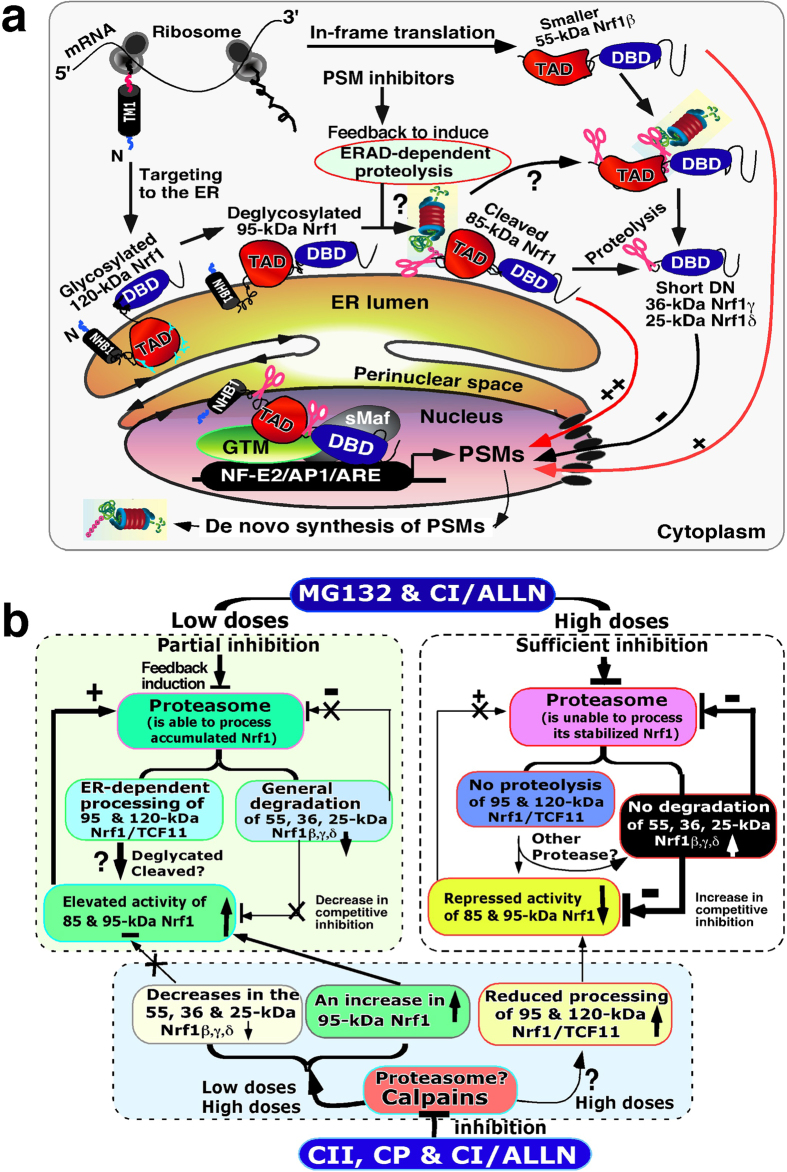
Figure 12. A bidirectional regulatory feedback circuit exists between Nrf1 and the 26S proteasome.
(a) A model is proposed, based on our findings (refs 23, 24, 25, 26,34,36 and this study) and those of others (refs 2,3,29, 30, 31,37,40), in order to explain the regulatory feedback circuit between Nrf1 and the 26S proteasome. The 120-kDa Nrf1 glycoprotein is retrotranslocated through the p97/VCP-driven pathway from the ER into the nuclo/cytoplasm where it is deglycosylated to yield an active 95-kDa isoform. The latter deglycoprotein is proteolytically processed to generate a cleaved 85-kDa Nrf1 isoform by the 26S proteasome that is activated by proteasome inhibitors through a feedback loop. In turn, transcriptional expression of the 26S proteasome and p97/VCP complexes is regulated by the 95-kDa and/or 85-kDa Nrf1 isoforms before the CNC-bZIP transcription factor is subjected to further proteasome-mediated processing that creates small Nrf1 isoforms of between 55-kDa and 25-kDa. Such small polypeptides may also be produced by in-frame translation of long Nrf1 mRNA transcripts and subsequently further degraded by the proteasome. (b) Schematic representation of a bidirectional regulatory feedback circuit exists between Nrf1 and the 26S proteasome. The transcriptional expression of 26S proteasomal subunits (e.g. PMSA4 and PMSB6) is regulated predominantly by Nrf1 (see refs 2,3,29, 30, 31,37,40). In turn, different doses of the proteasomal inhibitors MG132 and CI/ALLN exert opposing effects on the activity of Nrf1 to mediate expression of genes encoding proteasomal subunits. Specifically, at low doses, MG132 and CI/ALLN increase activity of Nrf1-target proteasomal subunit gene expressions by increasing the levels of the active 95- and/or 85-kDa Nrf1 isoforms, whereas high doses of MG132 and CI/ALLN inhibit Nrf1-mediated transcription by increasing the levels of the 36-kDa Nrf1γ and 25-kDa Nrf1δ dominant-negative isoforms. However, the detailed rational mechanism(s) of this bi-phasic response remains to be determined. In addition, further mechanistic study is warranted to identify which proteases enable the endoproteolytic processing of either 120-kDa or 95-kDa Nrf1 proteins to yield the cleaved 85-kDa isoform.