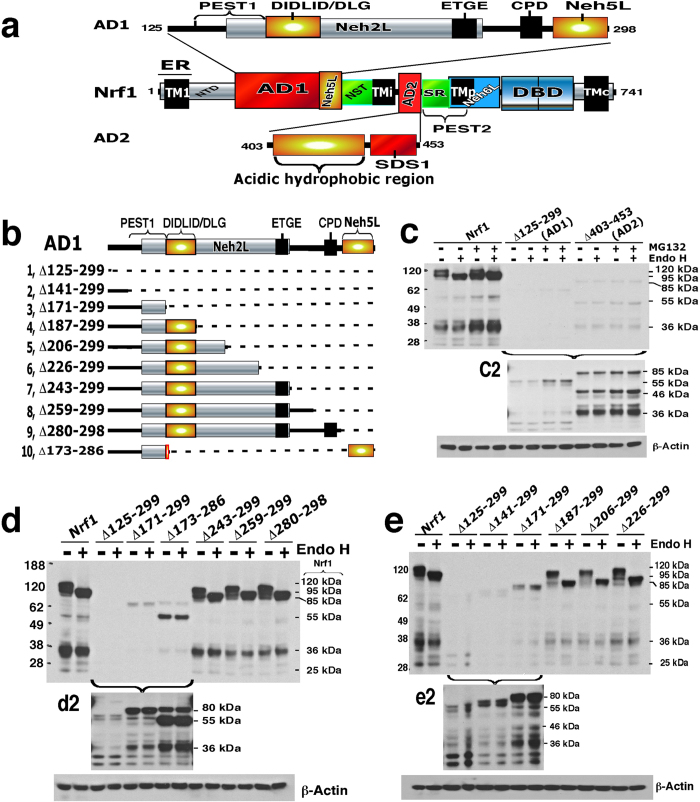
Figure 3. Nrf1 is differentially regulated by AD1, which is transiently translocated into the ER.
(a) Diagrammatic representation of discrete regions in AD1 and AD2, as well as several putative degrons in Nrf1. Within the CNC-bZIP factor, two acidic transactivation domains AD1 (aa 125–298) and AD2 (aa 403–453) are separated by the NST glycodomain (aa 299–400) that contains the TMi peptide (aa 376–393) on its C-terminal border. This location suggests that the functioning of AD1 and AD2 could be monitored by TMi, with the topological folding and positioning of TMi being controlled by glycosylation and the stress-dependent non-glycosylation and/or de-glycosylation of its adjacent peptide sequences. Furthermore, the TMp region (aa 507–525) is situated on the C-terminal border of the SR/PEST2 sequence (aa 454–519) that overlaps the Neh6L domain (aa 489–580), indicating that the topology of TMp could exert an opposing effect on Nrf1 activity, thereby bidirectionally regulating target genes. (b) A series of deletion mutants lacking various portions of AD1 (shown by hatched lines) are illustrated schematically. The locations of the PEST1 sequence (aa 141–170), the DIDLID/DLG element (aa 171–186) and the ETGE motif within the Neh2L subdomain (aa 156–242), CPD (i.e. 267LLSPLLT273) and the Neh5L subdomain (aa 280–298) are indicated. (c to e) COS-1 cells were transfected with expression constructs for wild-type Nrf1 or the indicated mutants, and were then allowed to recover from transfection for 24 h in 25 mM-glucose medium, prior to being treated with 5 μmol/l MG132 for an additional 2 h. Total cell lysates (30 μg protein) were subjected to deglycosylation reactions with 500 units of Endo H at 37 °C for 1 h before being stopped in denaturation buffer. Thereafter, the abundance of these proteins was determined by immunoblotting with V5 antibody; the film was exposed to the immunoblot for two different lengths of time, with the longer exposed film being cropped to eliminate intense bands (c2, d2 and e2). The masses of the Nrf1 isoforms was estimated to be 120, 95, 85, 55, 46, 36 and 25 kDa, and β-actin was employed as an internal control.