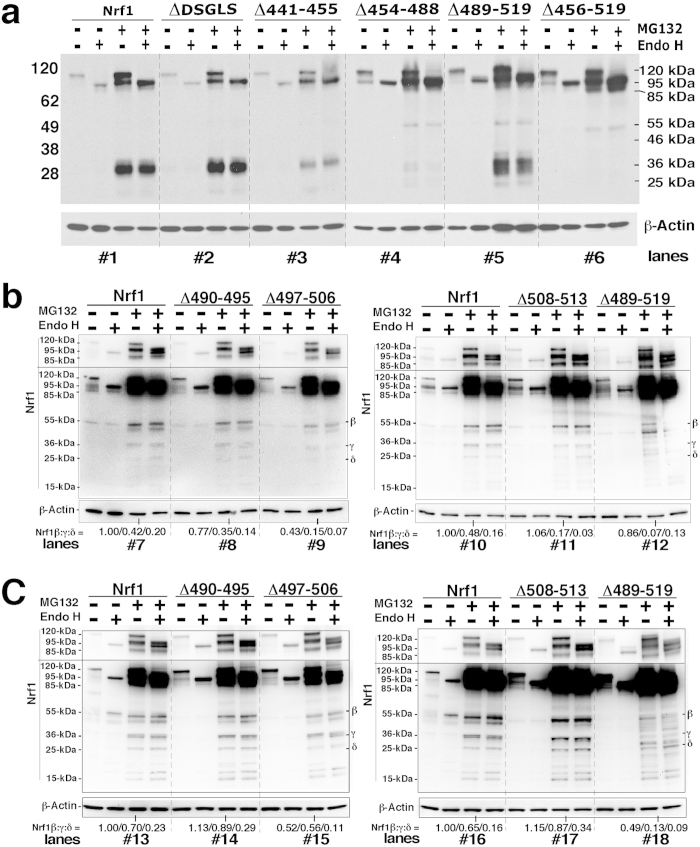
Figure 7. AD2-adjoining degrons selectively target Nrf1 for proteolytic processing into several small isoforms.
(a) When COS-1 cells were grown to reach ~90% of confluence, they were transfected overnight (for 18 h) with expression constructs for the intact wild-type Nrf1 or the indicated mutants lacking DSGLS, SDS1, SDS2, SR and PEST2, and then were allowed to recover from transfection for an additional 24 h in 25-mM glucose-containing medium before being treated with 5 μmol/l MG132 for 2 h prior to the termination of experiment. After in vitro deglycosylation reactions of denatured lysates with Endo H, the proteins were resolved using LDS/NuPAGE containing 4–12% Bis(polyacrylamide)-Tris gel in the pH 7.3 MES-SDS running buffer, and the blots probed with antibodies against the V5 epitope. The amount of proteins applied to each polyacrylamide gel sample well was adjusted to ensure equal loading of β-gal activity. In addition, β-actin was employed as an internal control of protein loading. (b and c) When COS-1 cells were grown to reach either ~50% (b) or ~70% (c) of confluences, they were transfected with expression constructs for the intact wild-type Nrf1 or the indicated mutants lacking SDS2 and its flanking peptides for 6 h (b) or 12 h (c). The cells were then allowed to recover from transfection for an additional 24 h in 25-mM glucose-containing medium before being treated with 5 μmol/l MG132 for 2 h. The total lysates were subjected to deglycosylation reactions, followed by protein separation by LDS/NuPAGE that contains 4–12% Bis-Tris gel in the pH 7.7 MOPS-SDS running buffers before being visualized by western blotting. The upper and middle images were presented for different times of exposure to the supersensitive reagent. Subsequently, the intensity of the 55-, 36- and 25-kDa protein blots was estimated by dividing the value for Nrf1 isoforms with that for β-actin, and then the relative amount of all relevant Nrf1 isoforms (detected on the same gel) was normalized to the basal level (designated 1.0) of the 55-kDa protein measured from MG132-treated cells expressing wild-type Nrf1, the results of which are shown in the bottom.