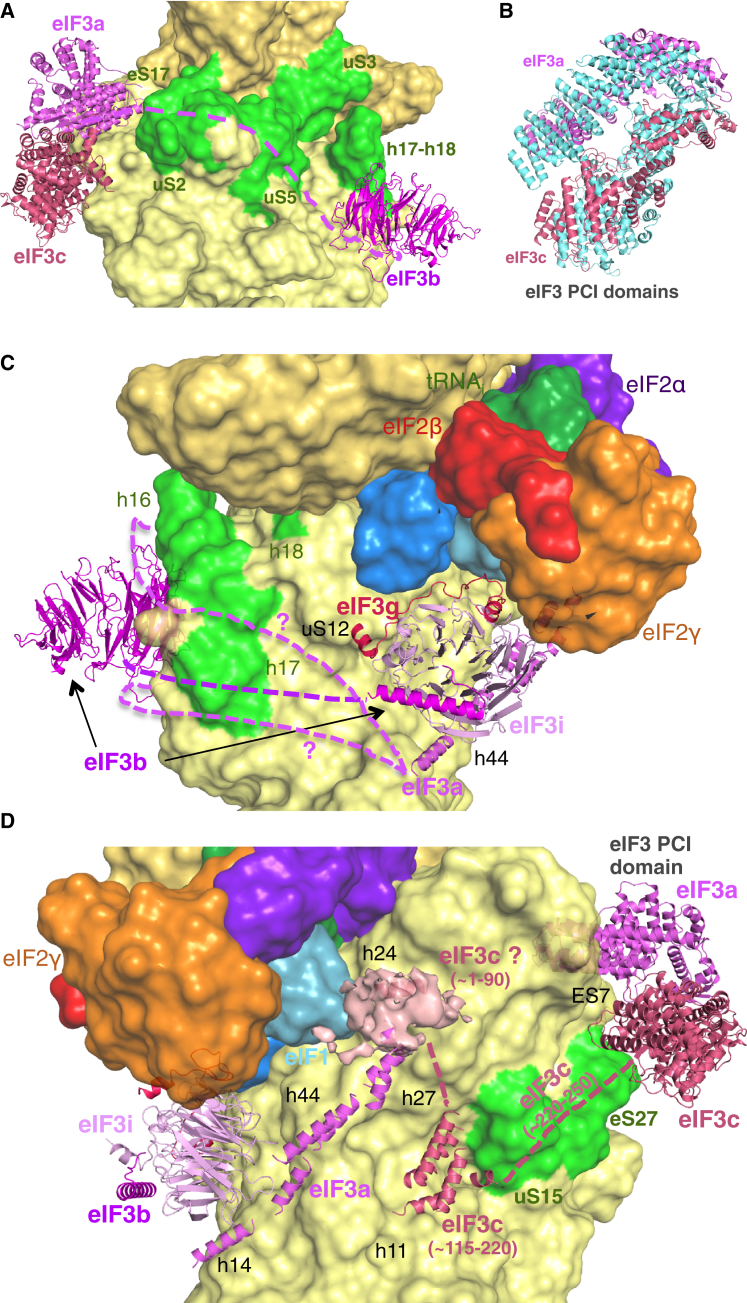
Figure 7.
Structural Arrangement of eIF3 Components in 48S PICs
(A) Locations of the eIF3a/eIF3c PCI domains and β-propeller of eIF3b at different positions on the solvent-exposed surface of the 40S, highlighting rRNA helices and ribosomal proteins (green) predicted to bind to eIF3a. The proposed path of the unassigned central portion of the eIF3a-CTD connecting the PCI domain to the subunit interface is shown as a dashed purple line.
(B) Lateral displacement of eIF3a/eIF3c PCI domains in py48S-closed versus their positions in yeast 40S•eIF1•eIF1A•eIF3 (PDB: 4UER).
(C) Trimeric eIF3b-CTD/eIF3i/eIF3g-NTD subcomplex is shown near h44 and interacting with eIF2γ and the 40S interface surface. The β-propeller of eIF3b is also shown. Two alternative proposed paths of the eIF3a-CTD connecting the PCI domain to the bundle of helices below the eIF3i β-propeller are shown as dashed purple lines.
(D) A cluster of helices tentatively assigned to eIF3c is located near h11 and uS15 (green). A globular density with a single modeled helix is tentatively assigned to the eIF3c-NTD in proximity to eIF1 and h24. The proposed path of a linker connecting the cluster of helices to the eIF3c PCI domain is shown as a dashed magenta line. Long helices tentatively assigned to eIF3a bridge the eIF3i β-propeller and h44 with the putative eIF3c-NTD and h24.