Figure 3.
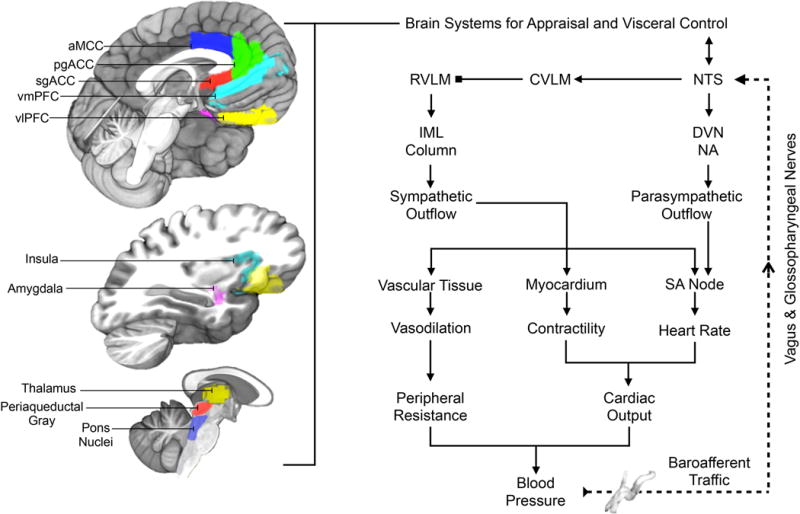
Illustration of selected brain areas engaged by psychological stressors and related to the expression of stressor-evoked cardiovascular (blood pressure and heart rate) responses, presumably via interactions with the baroreflex. On the left side of the figure are brain areas where stressor-evoked neural activity has been related to simultaneous changes in blood pressure, heart rate, and baroreflex measures (see text). Key areas include those presumably involved in mediating stressor appraisals and coordinating these appraisals with behavioral and physiological responding, including the dorsal anterior mid-cingulate cortex (aMCC), pregenual and subgenual areas of the anterior cingulate cortex (pgACC, sgACC), the ventromedial and ventrolateral prefrontal cortex (vmPFC, vlOFC), insula, and amygdala. These areas are networked with one another and with subcortical areas that play more proximal roles in the visceral regulation of cardiovascular function, including areas in the thalamus, periaqueductal gray, and pons. We note that this is not an exhaustive illustration of all brain areas involved in cardiovascular regulation. On the right side of the figure is a simplified illustration of the major motor and sensory limbs of the baroreflex, a homeostatic visceral control loop that regulates blood pressure. For clarity, not all components and connections are shown. Pressure sensitive cells (baroreceptors) that encode changes in blood pressure are concentrated in the major arteries (e.g., aorta, carotid artery). Their sensory signals (baroafferent traffic) are relayed via the vagal and glossopharyngeal nerves to a cell group in the medulla of the brainstem that influences both sympathetic and parasympathetic nervous system outflow: the NTS (nucleus of the solitary tract). The NTS not only projects to higher subcortical and cortical regions, but also to the dorsal vagal nucleus (DVN) and nucleus ambiguous (NA), which regulate parasympathetic outflow to the sinoatrial (SA) node of the heart to affect heart rate. The NTS also influences the rostral ventrolateral medulla (RVLM) indirectly via the caudal ventrolateral medulla (CVLM). The RVLM is a major source of excitatory drive to the sympathetic nervous system. Accordingly, indirect inputs from the NTS to the RVLM have the net effects of influencing sympathetic nervous system outflow to heart muscle (myocardium) to influence its contractility and the blood vessels (vascular tissue) to influence their level of dilation and resistance to blood flow in the periphery. These influences occur via RVLM projections to preganglionic cells of the sympathetic nervous system in the intermediolateral cell column (IML) of the spinal cord. Blood pressure itself is the product of the level of resistance to blood flow in the periphery and the volume of blood being pumped by the heart (cardiac output), which is determined by heart rate and myocardial contractility. Blocked endpoints denote inhibitory influences; arrowed endpoints denote excitatory influences. Broken lines correspond to ascending (afferent) visceral input, particularly from peripheral baroreceptors. The baroreflex is one example of a visceral control loop by which appraisal systems of the brain may link states of psychological stress and physiological responding to influence health.
