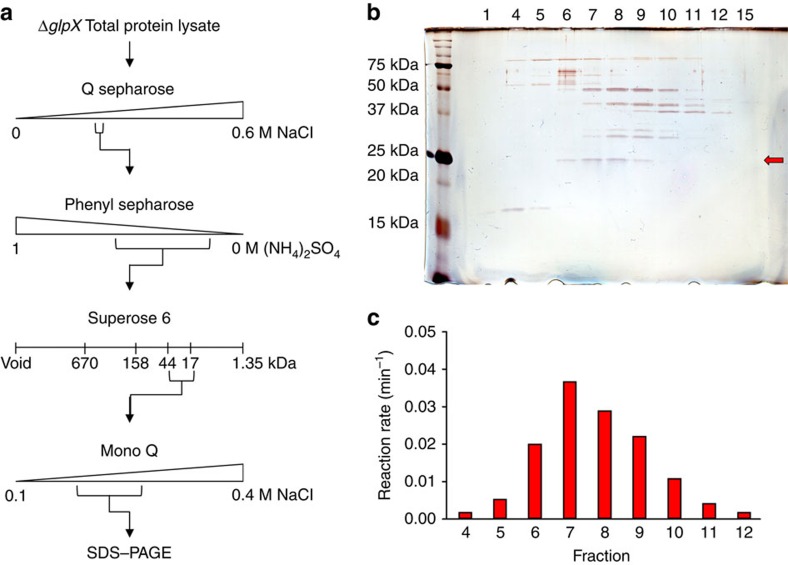
Figure 3. Identification of the second FBPase by biochemical extraction.
(a) FBPase activity of the ΔglpX cell lysate was purified using a series of liquid chromatography techniques followed by SDS–PAGE of the final active fractions. (b,c) Silver-stained SDS–PAGE gel (b) and FBPase activity profile using 12 mM FBP as substrate (c) for the range of fractions from the final Mono Q anion exchange chromatography with detectable FBPase activity. Inactive fractions 1 and 15 were included in the SDS–PAGE analysis. A single band of ∼25 kDa (red arrow in b) correlated with the FBPase activity profile of the fractions. Peptide mass fingerprinting identified the major protein component of this band to be GPM2 (Rv3214, molecular weight=21.95 kDa, 66.5% coverage, eight peptides).