Abstract
We used a murine model of type II diabetes, which reproduces the major features of the human disease, and a number of cellular models to study the antidiabetic effect of ANC, a standardised anthocyanin-rich formulation from maqui berry (Aristotelia chilensis). We also isolated delphinidin 3-sambubioside-5-glucoside (D3S5G), a characteristic anthocyanin from maqui berry, and studied its antidiabetic properties. We observed that oral administration of ANC improved fasting blood glucose levels and glucose tolerance in hyperglycaemic obese C57BL/6J mice fed a high fat diet. In H4IIE rat liver cells, ANC decreased glucose production and enhanced the insulin-stimulated down regulation of the gluconeogenic enzyme, glucose-6-phosphatase. In L6 myotubes ANC treatment increased both insulin and non-insulin mediated glucose uptake. As with the ACN, oral administration of pure D3S5G dose-dependently decreased fasting blood glucose levels in obese C57BL/6J mice, and decreased glucose production in rat liver cells. D3S5G also increased glucose uptake in L6 myotubes and is at least partially responsible for ANC’s anti-diabetic properties.
Keywords: Dietary Anthocyanins, Metabolic Syndrome, Glucose Metabolism, Maqui Berry
1. Introduction
The fruit from Aristotelia chilensis (Molina) Stuntz (Elaeocarpaceae), commonly known as maqui berry (MB), Chilean blackberry or “maqui” in Chile and Argentina, is a common wild, edible berry in central and southern Chile (Schreckinger, Lotton, Lila & de Mejia, 2010). MB has been recently reported as one of the healthiest exotic berries, due to its particularly high concentration of bioactive polyphenols (Cespedes, El-Hafidi M, Pavon N & J, 2008; Cespedes, Alarcon, Valdez-Morales & Paredes-Lopez, 2009; Escribano-Bailon, Alcalde-Eon, Munoz, Rivas-Gonzalo & Santos-Buelga, 2006; Schreckinger et al., 2010). Indeed, the fruit from A. chilensis display one of the highest ORAC antioxidant capacities (Cespedes et al., 2008; Céspedes, Valdez-Morales, Avila, El-Hafidi, Alarcón & Paredes-López, 2010; Morariou 2007) and anthocyanin concentration compared with other edible berries (Ruiz et al., 2005; Schreckinger et al., 2010) The polyphenolic profile of MB has been partially studied by different authors (Araya, Clavijo & Herrera, 2006; Cespedes et al., 2008; Escribano-Bailon et al., 2006; Miranda-Rottmann, Aspillaga, Perez, Vasquez, Martinez & Leighton, 2002; Ruiz et al., 2005; Schreckinger, Wang, Yousef, Lila & de Mejia, 2010.) and the major anthocyanins found in MB are glycosylated forms of delphinidin and cyanidin. Surprisingly, to date the biochemistry and health-promoting properties of MB have been scarcely studied. Evidence suggests that phenolic compounds extracted from MB display cardioprotective effects against ischaemia-reperfusion heart damage in mice (Cespedes et al., 2008). In line with this evidence, another set of data from in-vitro studies proposes that the anthocyanins from MB inhibit adipogenesis and inflammation (Schreckinger et al., 2010.), and prevent LDL oxidation (Miranda-Rottmann et al., 2002). A. chilensis has also been used in Chilean traditional medicine as antidiarrhoeic (Hoffman, 1991), anti-inflammatory and antipyretic (Cespedes, J, Avila & Nieto, 2010; Munoz, Barrera & Meza, 1981). MB’s potent antioxidant activity may be responsible for the inhibition of skin oxidative damage. (Crozier, Jaganath & Clifford, 2009). Several groups including ours have reported that dietary polyphenolics (Al-Awwadi et al., 2004a; Al-Awwadi et al., 2004b), including anthocyanins from berries, display potent antidiabetic effects (Grace et al., 2009; Tsuda, Horio, Uchida, Aoki & Osawa, 2003) and represent a promising source of functional foods with antidiabetic properties. Interestingly, even though MB has a particularly high concentration of anthocyanins, the effect of MB’s anthocyanins on glucose metabolism and insulin resistance has not yet been researched.
The physiological effects of anthocyanins from fruits have been questioned, due to their limited bioavailability after oral intake (Crozier et al., 2009; Wilson, Meyers, Singh, Limburg & Vorsa, 2008). Our group recently reported that the antidiabetic effects of orally administered anthocyanins from blueberries can be significantly increased by using self-microemulsifying agents, such as Labrasol (LAB) (Grace et al., 2009).
In this work we report for the first time the antidiabetic effect of an anthocyanin-rich formulation from MB on diet-induced obese hyperglyacemic mice and analysed the effect of the bioenhancer Labrasol on orally administered MB anthocyanins. We also isolated and characterised delphinidin 3-sambubioside-5-glucoside (D3S5G) as one of the main bioactive anthocyanins responsible for the antidiabetic effect of MB. We used several in-vitro models of type II diabetes to explore potential target tissues of MB anthocyanins.
2. Materials and methods
2.1 Chemicals
2-deoxy-[3H]-D-glucose was purchased from GE Healthcare (Amersham, UK). Dexamethasone, 8(4-chlorophenylthio)-cAMP, sodium lactate, and sodium pyruvate were purchased from Sigma-Aldrich (St. Louis, MO). Human insulin (Humulin) was purchased from Eli Lilly (Indianapolis, IN). All other chemicals, including cell culture media, were obtained from Invitrogen (Carlsbad, CA). Reagents for qPCR experiments were obtained from Applied Biosystems (Foster City, CA). The H4IIE (CRL-1548) cell line was obtained from American Type Culture Collection (Manassas, VA). L6 Myoblast cell line was a kind gift from Dr. Philip Blian at The Hospital for Sick Children, Toronto, Ontario, Canada. Pepsin A from porcine stomach mucosa (2500−3500 units/mg, P-7012), trypsin from bovine pancreas (7500 N-α-benzoyl-L-arginine ethyl ester (BAEE) units/mg, T9201), and α-amylase, Type II-A from Bacillus species (1333 units/mg A-6380) were obtained from Sigma-Aldrich (Stockholm, Sweden); fresh pig bile was provided by TNO (Zeist, Netherlands), and Rhizopus lipase (150,000 units/mg F-AP-15) was obtained from Amano Enzyme Inc. (Nagoya, Japan). Cyanidin3-O-glucoside and delphinidin 3-O-sambubioside standards were obtained from Polyphenols Laboratories (Sandnes, Norway). All other chemicals were obtained from Sigma-Aldrich (Stockholm, Sweden).
2.2 Preparation of anthocyanin-enriched formulations
Freeze-dried MB were obtained from Fundación Chile, Santiago, Chile. Anthocyanin-enriched formulations were prepared as described elsewhere (Grace et al., 2009; Ribnicky et al., 2009). Briefly, the freeze-dried fruits were extracted using 70% methanol that resulted in crude extract (CE). This CE was further cleaned up through an Amberlite XAD-7 column. The polyphenolics were absorbed on the Amberlite resin, which was preconditioned with acidified water (0.3% trifluoroacetic acid). The resin was washed thoroughly with acidified water to remove free sugars, pectins and phenolic acids. The polyphenolic mixture was then eluted with 100% methanol. This last fraction, named PAE, was further purified by Sephadex LH-20 column chromatography using 20% methanol in water to obtain the anthocyanin-enriched fraction (ANC).
2.3 Quantitative analysis of anthocyanins in MB formulations
The concentrations of anthocyanins in CE, PAE, and ANC were determined by HPLC using commercial anthocyanin standards. The HPLC analysis was carried out using an Agilent 1200 Series instrument (Agilent Technologies, Inc., Santa Clara, CA), equipped with autosampler and photo diode array detector (PDA). The mobile phase was 5% formic acid in H2O (A) and 100% methanol (B). The flow rate was constant at 1 mL/min. Solvent gradient was 10%, 15%, 20%, 25%, 30%, 60%, 10%, and 10% of solvent B at 0, 5, 15, 20, 25, 45, 47, and 60 min, respectively. Samples were filtered through 0.2-μm PTFE filters (Fisher Scientific, Pittsburgh, PA). For each sample, in three replicates, 15 μL was injected into the HPLC column (Supelcosil-LC18 column, 25 mm × 4 mm × 5 μm; Supelco, Bellefonte, PA). The absorption was measured at a wavelength of 520 nm. Using anthocyanin commercial standard, cyanidin 3-O-glucoside, the concentration of anthocyanins in MB crude extract (CE) and enriched fractions (PAE, ANC) were estimated as mg/g.
2.4 Glucose metabolism in obese hyperglycaemic mice
All procedures involving mice were conducted in strict compliance with relevant state and federal laws, the Animal Welfare Act, Public Health Services Policy, and guidelines established by the Institutional Animal Care and Use Committee under the active protocol number 04–023 at Rutgers University. We used a standard diet-induced murine model of type II diabetes (Surwit, Kuhn, Cochrane, McCubbin & Feinglos, 1988). These C57BL/BJ mice carry a genetic predisposition to develop characteristic features of type II diabetes when fed a high fat diet (Lin, Thomas, Storlien & Huang, 2000). Thus, six-week-old male C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME), housed on an artificial 12 h light and dark cycle and maintained on a high fat diet (HFD) containing 60% fat-derived calories (D12492 [GenBank], Research Diets) for 12 weeks. For the evaluation of acute hypoglycaemic effect we used the method described elsewhere (Grace et al., 2009) with minor modifications. Briefly, after 13 weeks on the HFD, the mice were randomised into different groups (n = 4−6). The general experimental set up was as follows: (i) a control group gavaged with a vehicle solution, which was either water or a mixture of Labrasol/water: 66/34 (LAB); (ii) one or more treated groups gavaged with either one or more of the following treatments: ANC, Labrasol/water: 66/34+ANC (LAB+ANC), D3S5G, and metformin, at the required doses.
After gavage, plasma glucose levels were measured in blood samples taken from the tail vein at the specified time points from 4 to 6 h. To perform the glucose tolerance test we used the method described elsewhere (Kizelsztein et al., 2009). Briefly, 20-week-old HDF mice were grouped into two groups of ten mice per group, fasted overnight (16 h) and then gavaged with 2.0 g/kg glucose solution. MB anthocyanins-enriched formulation was orally administered 2 h before glucose gavage. Plasma glucose levels were measured immediately before, 30, 60, and 120 min after the glucose challenge. In all the animal experiments blood glucose levels were measured using a glucometer (Lifescan; Johnson and Johnson, New Brunswick, NJ).
2.5 Cell Cultures
H4IIE hepatoma cells were incubated in 24-well plates (Greiner Bio-One, Monroe, NC) and grown to near confluency in Dubecco’s Modified Eagle Medium (DMEM), containing foetal bovine serum (FBS) 10% (v/v). Penicillin and gentamicin were always added to the media at concentrations of 100 IU/mL and 50 μg/mL, respectively. For gene expression experiments, H4IIE cells were treated for 8 h with 500 nM dexamethasone and 0.1 mM 8-CTP-cAMP (Dex-cAMP), to induce G6P gene expression, together with different concentrations of anthocyanins or anthocyanins + insulin. Three wells were allocated for each experiment, including one negative control (untreated cells) and one positive control (cells treated with insulin). L6 myoblasts from rat skeletal muscles (Loike, Zalutsky, Kaback, Miranda & Silverstein, 1988) were plated in 1.9 cm2 well uncoated of a 24-well plate (CELLSTAR, Grainer Bio-One) at a density of 40,000 cells/well and incubated at 37 °C in 5% CO2, 95% air in a medium composed of 10% (vol/vol) FBS and DMEM with 5 mM glucose until confluence was reached, which normally occurred within 2−3 days. DMEM medium was changed every 24 h. Penicillin and gentamicin were added to DMEM to a concentration of 100 IU/mL and 50 μg/mL, respectively. Once 80−95% confluency was reached, cells were differentiated using a standard protocol as described elsewhere (Loike et al., 1988). Briefly, cell differentiation was induced by sequential incubation of L6 myoblasts in DMEM with 2% (vol/vol) horse serum, and 100 nM insulin for 48 h. Then media was replaced with DMEM with 2% (vol/vol) horse serum for another 24 h. After the differentiation program, cells were immediately used for glucose uptake experiments. The percentage of myotube formation was determined as the percentage of nuclei present in multinucleated myotubes phase-contrast microscopy and Giemsa staining. The differentiation protocol used was also validated by evaluation of insulin sensitivity. In all the experiments 80–90% of the myoblasts fused into myotubes. All the experiments were performed on cells from passage eight or less.
2.6 Glucose Production Assay
H4IIE rat hepatoma cells were serum starved for 16 h in the glucose production buffer (glucose-free Dulbecco’s modified essential medium, pH 7.4, containing 20 mM sodium lactate and 2 mM sodium pyruvate without phenol red) and subsequently treated for 8 h with 2 mM metformin or different concentrations of ANC or D3S5G. At the end of the incubation period, 0.5 mL of medium were taken out to measure the glucose concentration in the culture medium using the Amplex Red® glucose assay kit (Invitrogen) according to manufacturer instructions. All the experiments were performed with cells from passage ten or less. Corrections for cell number were made on the basis of the protein concentration measured using the BCA Protein assay kit (Pierce Biotechnology, Rockford, IL). Results were expressed as folds control.
2.7 Total RNA Extraction, Purification and cDNA Synthesis
Total RNA was extracted from H4IIE cells using Trizol reagent (Invitrogen) following manufacturer instructions. RNA was quantified spectrophotometrically by absorbance measurements at 260 nm and 280 nm using the NanoDrop system (NanoDrop Technologies, Wilmington, DE). Quality of RNA was assessed by separation in gel electrophoresis. To remove any traces of DNA contamination, RNA was then treated with Dnase I (Invitrogen) following the manufacturer guidelines. The cDNAs were synthesised using 3.0 μg of RNA for each sample, using Applied Biosystems High Capacity cDNA Reverse Transcription Kit following the manufacturer’s protocol.
2.8 Quantitative PCR analysis of H4IIE rat Hepatoma Cells
The qRT-PCR (qPCR) amplifications were carried out in triplicates on an ABI 7300 Real-Time Detection System in a total volume of 25 μL containing 12.5 μL Brilliant SYBR® Green PCR master mix (Applied Biosystems), 5 μL of the 1:25 diluted cDNA, 0.5 μL of 6μM gene-specific primers (IDT, Coralville, IA) and 7 μL PCR-grade water. The primers were selected using the Primer Express Version 2.0 software (Applied Biosystems) as follows: β-actin (NM_031144), forward primer 5′-GGG AAA TCG TGC GTG ACA TT-3′, reverse primer 5′-GCG GCA GTG GCC ATC TC-3′; G6P (NM_013098.2), forward primer 5′-TCT ACC TTG CGG CTC ACT TTC-3′, reverse primer 5′-GAA AGT TTC AGC CAC AGC AAT G-3′. qPCR amplifications were performed on the 7300 Real Time PCR System (Applied Biosystems) using 1 cycle at 50 °C for 2 min, 1 cycle at 95 °C for 10 min, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The dissociation curve was completed with one cycle of 15 s at 95 °C, 1 min at 60 °C, and 15 s at 95 °C. NRT (no RT) and NTC (no template control) were included in each experiment as quality control steps. Target mRNA expression was analysed using the ΔΔCt method and normalised with respect to the expression of the β-actin housekeeping gene (Kizelsztein et al., 2009). The Dex-cAMP treatment (positive control) served as the calibrator sample in this study, and a value of 1.0 was assigned to the target gene expression of the calibrator sample. All samples were run in triplicate. A p-value of 0.05 was considered to be significant.
2.9 2-Deoxy-[3H]-D-glucose uptake
The cells grown and differentiated in 24-well plates were incubated in FBS-free DMEM for 6 h in the presence or absence of the experimental treatments (vehicle, ANC, D3S5G or insulin). The cells were then rinsed twice with KRPH (HEPES-buffered Krebs-Ringer phosphate), consisting of 118 mM NaCl, 5 mM KCl, 1.3 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, and 30 mM HEPES (pH 7.4). The uptake of the 22 nM 2-deoxy-[1-3H] glucose (2-DG) at 0.2 μCi/mL, was measured over 15 min. All the uptake measurements were made in triplicate. In these conditions, the nonspecific uptake was determined in the presence of 10 μM Cytochalasin-B. The uptake of 2-DG was terminated after 15 min by rapidly aspirating off the radioactive incubation medium and washing the cells two times in ice-cold phosphate-buffered saline. The radioactivity associated with the cells was determined by cell lysis in 0.05% Triton X-100, followed by the addition of 3 mL of liquid scintillation cocktail (Liquid Scintillation Cocktail, Beckman Coulter, Inc., Fullerton, CA). The mixture of cell lysate and scintillation cocktail was vortexed for 20 s, incubated in darkness for 16 h and radioactivity was measured in a LS6500 multipurpose scintillation counter (Beckman Coulter, Inc.). In these experimental conditions, nonspecific glucose uptake was 10% of the total uptake. For all the experiments, corrections for cell number were made on the basis of the protein concentration measured using the BCA protein assay kit (Pierce Biotechnology), according to manufacturer instruction. Glucose uptake results were expressed as fold negative control.
2.10 Cell Cytotoxicity
Alterations of the cell membrane integrity and potential cytotoxic effects of MB anthocyanins to L6 myotubes and H4II cells were studied. For this purpose, cells were seeded at 40 × 103 cells/well in a 24-well plate and allowed to attach for 24 h. Subsequently, ANC and D3S5G were added to the medium at various concentrations (0 to 100 μg/mL). After 16-h treatment, membrane integrity and cell viability were assessed by Calcein-AM (Ca-AM) incorporation. Ca-AM was added to each well at 3μM, and incubated for 1 h at 37 °C, and then fluorescence was measured in a plate reader (BIOTEK Synergy II, Biotek Instruments Inc, Winooski, VT) with excitation wavelength at 485 nm and emission wavelength of 525 nm. Triplicates were used for each experimental condition.
2.11 Isolation and Purification of Anthocyanins from ANC
Delphinidin 3-sambubioside-5-glucoside (D3S5G), one of the major anthocyanins of MB, was purified from ANC by fast centrifugal partition chromatography (FCPC) using an A1000 FCPC instrument (Kromaton Technologies, Angers, France), equipped with a 1-L rotor. The solvents were delivered by a binary gradient pump (ASI model 501). A UV-Visible single wavelength detector (Reflect Scientific model VUV-24) was used to monitor the absorbance of the eluting compounds. The sample collection system included a micro-computer controlled fraction collector (Advantec Toyo Kaisha Ltd., model CHF122SC), with test tubes and prep. funnel racks. The data acquisition system included Clarity Lite v. 2.4.4.105 software from Data Apex (Prague, Czech Republic). The FCPC solvent system used for separation consisted of ethyl acetate, n-butanol and water in a ratio of 2:3:5 with 0.1% trifluoroacetic acid as described previously (Degenhardt, Knapp & Winterhalter, 2000) with minor modifications. Each component of the solvent system was added to a 4-L separatory funnel in a volume ratio of 2:3:5 and equilibrated at room temperature for 20 min. The upper phase and the lower phase were separated. The lower phase was used as stationary phase and the upper phase as mobile phase. The FCPC system was loaded with 400 mg of ANC. During 200 min, fractions were eluted and collected from the rotor. The detector was set to 520 nm and the separation process was monitored by absorbance at this wavelength. The fractions containing D3S5G were confirmed by LC-MS and subjected to two steps of purification using preparatory HPLC. The LC/MS system included a Waters (Milford, MA) W616 pump, a W600S controller, W717plus auto-sampler, and Waters W996 photodiode array (PDA) detector. UV data were collected & analysed with the Waters Millennium® v. 3.2 software. After the 996 PDA detector the eluent flow was guided to a Varian 1200L (Varian Inc., Palo Alto, CA) triple quadrupole mass detector with electrospray ionisation interface (ESI), operated in positive ionisation mode. The electrospray voltage was adjusted to 5 kV and sheath gas was nitrogen; the mass detector was used in scanning mode from 65 to 1500 atomic mass units (amu). Data from the Varian 1200L mass detector was collected, compiled and analysed using Varian’s MS Workstation, Version 6.41, SP2. Substances were separated on a Phenomenex® Luna C-8 reverse phase column, size 250 × 4.6 mm, particle size 5 μm, pore size 100 Å, equipped with a Phenomenex® SecurityGuard™ pre-column. The mobile phase consisted of 2 components: solvent A (0.5% acetic acid in double distilled de-ionized water, pH 3–3.5), and solvent B (100% acetonitrile). The mobile phase flow was 0.2 mL/min, and a gradient mode was used for all analyses. The initial conditions of the gradient were 85% A and 15% B; for 30 minutes the proportion reached 5% A and 95% B which was kept for the next 3 minutes; from minute 38 to minute 41 the gradient went back to initial conditions. A 10-minutes equilibration interval was included between subsequent injections. The purification of D3S5G from FCPC fractions was done by a Waters preparative HPLC system consisting of W717 plus autosampler, W600 multi-solvent delivery system controller, W616 pump with preparative head, W490E multi-wavelength detector and Waters fraction collector. Data was collected and analysed using Waters Millennium v.32 software. For the first step of the preparative HPLC, a Phenomenex®, 250 × 21.2 mm Synergi Max reverse phase column with a particle size of 10μm was used. The mobile phase consisted of two solvents: solvent A (water:acetonitrile:formic acid in the ratio 8.7:0.3:1) and solvent B (water:acetonitrile:formic acid in the ratio of 4:5:1). A gradient elution of 6% B to 20% B, over 100 minutes was used at a flow rate of 10.5 ml/min. In the second step of purification, a Phenomenex®, 250 × 4.6 mm Synergi Max reverse-phase column with a particle size of 4 μm was used. The mobile phase consisted of solvent A (0.5% acetic acid in water) and solvent B (acetonitrile). A gradient elution of 5% B to 95% B, over 70 minutes, was used at a flow rate of 1 mL/min. Identity of the isolated D3S5G was confirmed by NMR analysis, which was run on a Bruker Advance III 950 MHz spectrometer, in solvent CD3OD, and TMS as internal standard.
2.11 Statistical Analysis
The data from in-vitro tests was analysed using ANOVA, and Bonferroni’s multiple comparison test. The data from the animal experiments was analysed using Student’s t-test; p < 0.05 was considered significant.
3. Results
3.1 Qualitative and quantitative analysis of anthocyanins in MB formulations
The anthocyanin formulations (CE, PAE and ANC) prepared by sequential column purification from freeze-dried MB, according to the protocol described in this paper, were characterised by ESI-LC-MS in the positive ion mode and the major anthocyanins were identified as: delphinidin 3-sambubioside-5-glucoside (m/z: 759), delphinidin 3,5-diglucoside (m/z: 627), cyaniding 3-sambubioside-5-glucoside (m/z: 743), cyaniding 3,5-diglucoside (m/z 611), delphinidin 3-sambubioside (m/z: 597), delphinidin 3-glucoside (m/z: 465), cyanidin 3-sambubioside (m/z: 581), and cyaniding 3-glucoside (m/z 449). The total anthocyanin content of each formulation, as quantified by HPLC, was higher following each enrichment step (39.5, 391.5 and 614.3 mg/g, for CE, PAE and ANC, respectively) (Fig. 1 and Table 1).
Figure 1. Identification of delphinidin 3-sambubioside-5-glucoside.
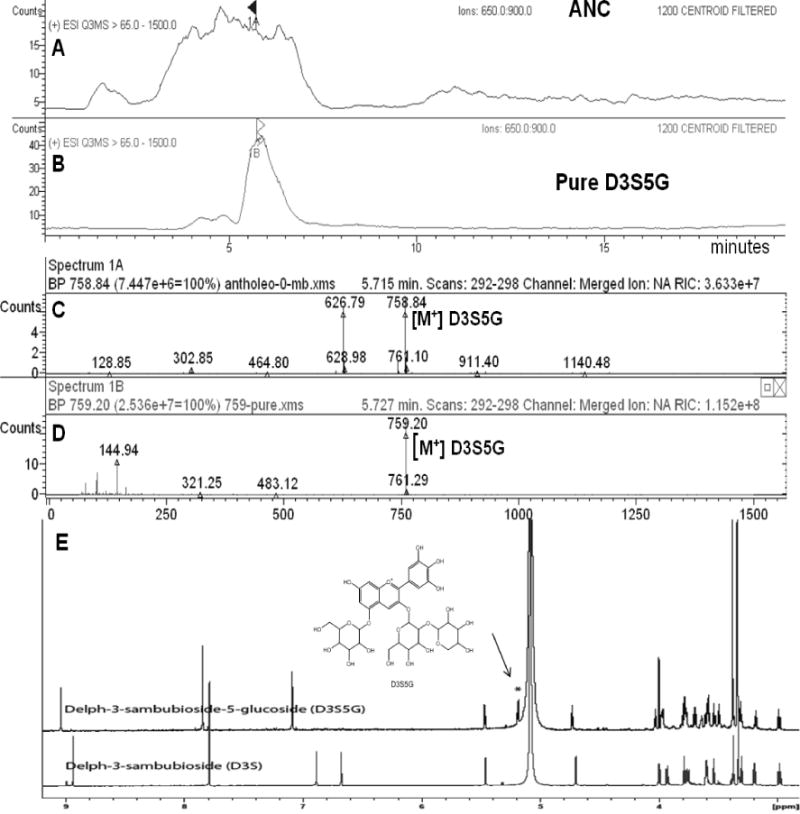
LC-MS showing the extracted ion chromatograms in the range of m/z 650 to 900 of: (A) ANC, (B) D3S5G; (C) Mass spectrum (positive ion mode) extracted at retention time 5.7 min of ANC fraction showing mainly m/z 758.8 (D3S5G) and m/z 626.79 (D3G,5G); (D) Mass spectrum of the purified D3S5G m/z 759.2. (E) 1H-NMR spectrum of isolated D3S5G compared to reference standard D3S measured at 950 MHz. D3S5G shows an extra signal at δ 5.2 for the anomeric proton of the 5-glucose.
Table 1.
Individual and total anthocyanin concentrations in CE, PAE and ANC fraction
| Anthocyanin | Crude (CE) |
Post Amberlite (PAE) |
Post Sephadex (ANC) |
|---|---|---|---|
| Delphinidin 3-sambubioside-5-glucoside | 3.2 | 36.2 | 67.6 |
| Delphinidin 3,5-diglucoside | 3.3 | 37.2 | 65.0 |
| Cyanidin 3-sambubioside-5-glucoside + Cyanidin3,5-diglucoside | 2.2 24.5 | 44.8 | |
| Delphinidin 3-sambubioside | 8.8 | 85.6 | 127.0 |
| Delphinidin 3-glucoside | 13.5 | 125.0 | 192.1 |
| Cyanidin 3-sambubioside | 0.2 | 2.8 | 4.3 |
| Cyanidin 3-glucoside | 8.3 | 80.2 | 113.5 |
| Total (mg/g) | 39.5 | 391.5 | 614.3 |
3.2 Structural Characterisation of D3S5G
LC-ESI-MS analysis, in positive ion mode, of the purified anthocyanin showed a molecular ion peak at 759 [M]+ corresponding to a molecular formula C32H39O21. ESI-MS-MS showed fragment ions at m/z 627 [M − xylose], m/z 465 [M − glucose − xylose] and the distinct MS-MS fragment m/z 303 for the aglycone delphinidin, after the loss another glucose unit. 1H-NMR analysis (CD3OD, 950 MHz) of the purified anthocyanin showed a downfield aromatic singlet at δ 7.8, integrated for two protons and assigned for H2′ and H6′, indicating trihydroxy substitution of the flavonoid-ring B. The aromatic protons at positions 6 and 8 appeared overlapped as one broad singlet at δ 7.8. Three anomeric protons illustrating three sugars displayed three doublets at δ 5.46 (J = 7.6), 5.18 (J =7.7) and 4.72 (J = 7.7) for 3-O-glucose, 5-O-glucose and xylose respectively. The large coupling constants for the three anomeric protons indicated β-sugar linkage.
Comparing the 1H NMR spectra of the isolated anthocyanin with the 1H NMR spectra of standard delphinidin 3-sambubioside (glucose-xylose) indicated close similarity in the higher field region, except for the additional anomeric proton of the 5-O-glucose moiety (see Fig 1 E). Also there were additional signals assigned for the rest of the glucose protons in the region between 2.9 and 4.05 ppm. The study of the homo-and heteronuclear NMR spectra confirmed the structure of the isolated anthocyanin to be delphinidin 3-O-sambubioside-5-O-glucopyranoside. D3S5G has been previously identified in MB by HPLC-MS (Escribano-Bailon et al., 2006; Schreckinger et al., 2010), but never isolated from the fruits of Aristotelia chilensis.
3.3 Glucose metabolism in obese hyperglycaemic mice
Hypoglycaemic effects of MB extracts were evaluated in hyperglycaemic obese mice 6 h after oral administration (Fig. 2). The hyperglycaemic obese C57BL/6J male mice were fed a high fat diet for 16 weeks with 60% of the caloric content contributed by fat (lard). Mice were treated once with different fractions of MB and the glucose content in blood was analysed. The ANC preparation produced a significant reduction in fasting glucose levels while PAE preparation had a less potent effect that was not statistically significant and the CE treatment did not change blood glucose levels compared to vehicle-treated animals (Fig. 2A). In addition, ANC dose-dependently lowered blood glucose levels of diet-induced insulin resistant mice at doses ranging from 50−500 mg/kg and this effect occurred at 4 h after oral administration with doses of 125 mg/kg and higher (Fig. 2C). Maqui anthocyanins were active after oral administration even without the bioenhancer Labrasol (Fig. 2B). The acute hypoglycaemic effect of ANC was compared to metformin, a standard therapeutic agent for Type II diabetes, which was used experimentally as a positive control in these experiments. A glucose tolerance test was performed following a 2-h pre-treatment of HFD mice with ANC fraction ANC improved glucose tolerance in obese hyperglycaemic mice fed high-fat diet (Fig. 2D). The average blood glucose levels of ANC-treated mice and untreated mice after 60 min of oral glucose (2 g/kg) were 266 and 364 mg/dL, respectively.
Figure 2. Effect of MB anthocyanins on the glucose metabolism of diet-induced obese hyperglycaemic mice.
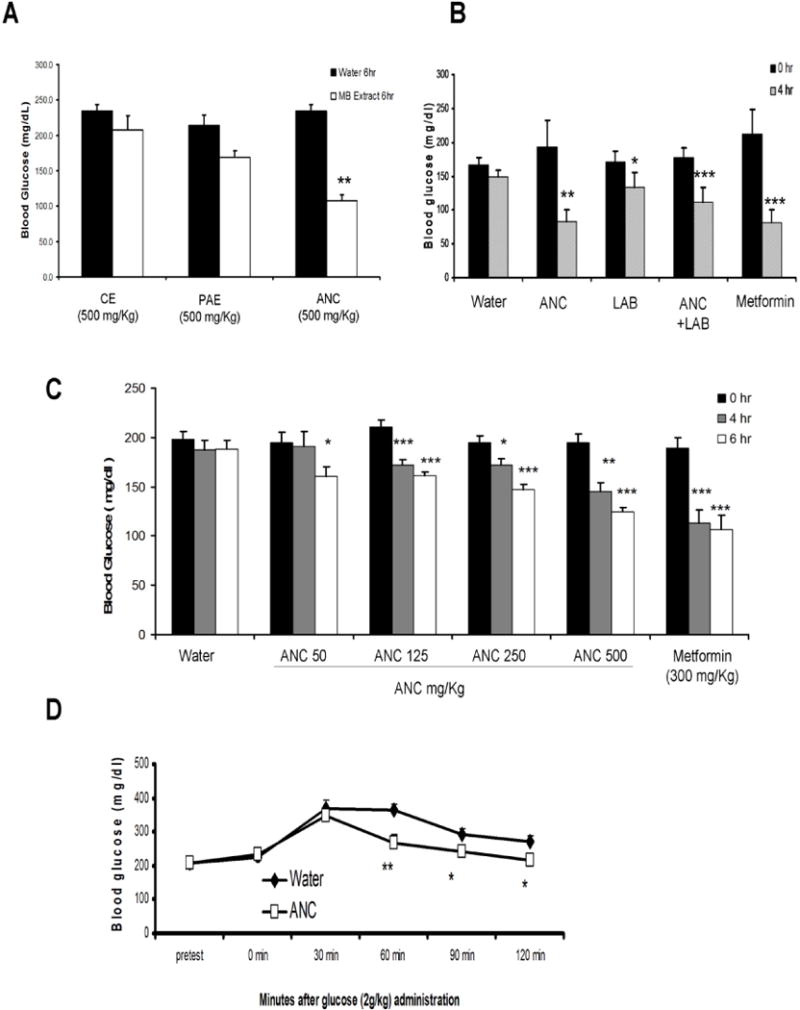
(A) Fasting blood glucose levels of mice measured 6 h after oral administration of vehicle (water) or different preparations of MB (CE = Crude Extract; PAE = Post Amberlite Extract, ANC = Anthocyanin-Rich Fraction) at 500 mg/kg. (B) Fasting blood glucose levels of mice measured 4 h after oral administration of water, ANC at 500 mg/kg (ANC), ANC at 500 mg/kg with Labrasol 66% (ANC+LAB), and metformin at 300 mg/kg. (C) Changes in the fasting blood glucose levels of hyperglycaemic mice at 4 and 6 h after oral administration of ANC over a dose range (50−500 mg/kg). Water and metformin (300 mg/kg) were included as control groups. In graphs A, B, and C results are the mean of n = 4, +/− SD, *: p < 0.05, **: p < 0.01, ***: p < 0.001 vs 0 h. (D) Effect of the oral administration of ANC at 500 mg/kg on the glucose tolerance of hyperglycaemic obese mice. Blood glucose levels were determined in these mice every 30 min for 120 min beginning 30 min before glucose gavage. Values are mean of n = 10 +/− SD, *: p < 0.05, **: p < 0.01
3.4 Effect in glucose production and gene expression in liver cells
The inadequate metabolism of glucose in the liver may significantly contribute to the development of insulin resistance and type II diabetes (Kizelsztein et al., 2009; Wolfrum, Asilmaz, Luca, Friedman & Stoffel, 2004). Therefore, the effect of ANC on glucose production was determined in H4II rat hepatoma cells, a validated disease-relevant model of type II diabetes (de Raemy-Schenk et al., 2006). H4IIE cells were starved for 16 h in DMEM glucose-free medium and subsequently treated with different concentrations of MB anthocyanins or metformin. ANC significantly decreased glucose production (Fig 3A). No toxic effects were observed in any of the treatments. The effect of ACN on G6P gene expression was investigated as a possible mechanism underlying the decrease of glucose production. We observed that treatment of H4IIE cells with ANC at concentration of 50 μg/mL produced only a mild downregulation of G6P gene relative to insulin (Fig. 3B). When ANC was provided at 20 μg/mL along with a sub-optimal dose of insulin, it produced an insulin sensitising effect in the in H4IIE cells, which was evidenced by a significant increase in the downregulation of the G6P enzyme gene (Fig. 3C).
Figure 3. Effect of MB anthocyanins on glucose production and gene expression of G6P in H4IIE cells.
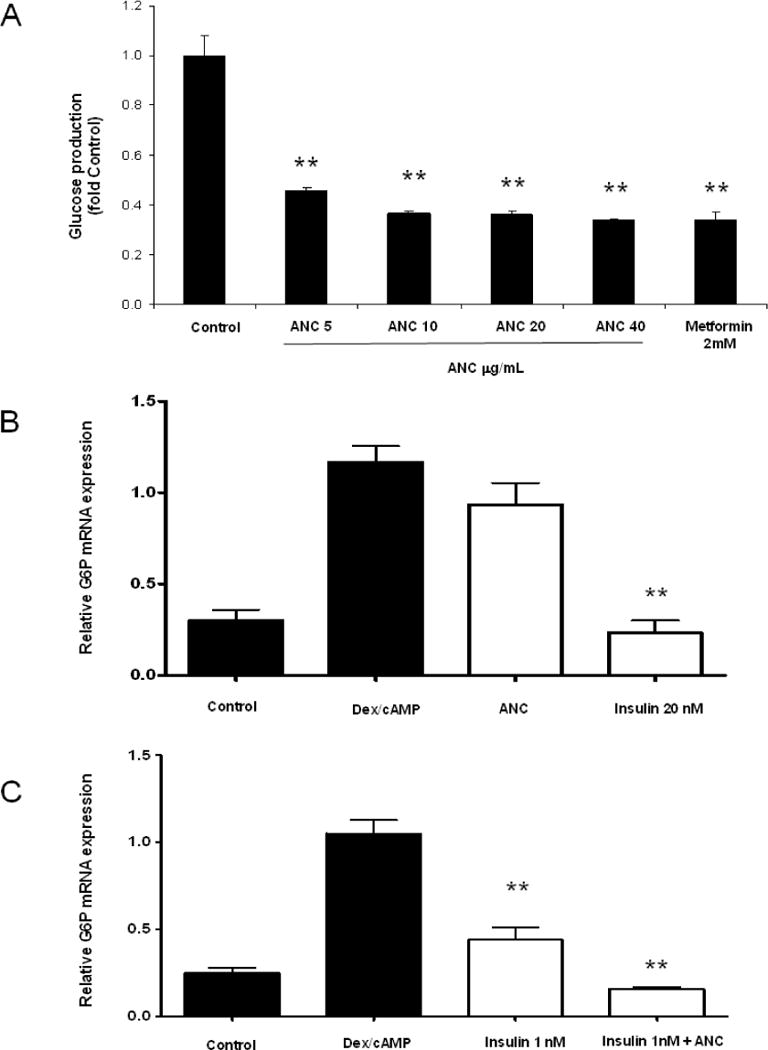
(A) Glucose production was measured in H4IIE cells after 8 h of treatment with ANC at doses of 0−40 μg/mL or metformin as a positive control at 2mM. Data represent the mean of three experiments +/− SD. (B) GP6ase mRNA expression was evaluated in H4IIE cells treated with Dex-cAMP (except for negative control) after 8 h of treatment with ANC at 50 μg/mL or 20 nM insulin. (C) An insulin-sensitising effect over G6P was evaluated in H4IIE cells treated with Dex-cAMP (except for negative control) after 8 h of treatment with ANC at 20 μg/mL together with insulin at 1 nM relative to insulin alone at 1 nM. G6P was normalised to β-actin mRNA and the specific fold changes were calculated as a ratio of the target mRNA level relative to the response to Dex-cAMP activation. In A, B and C data represent the mean of three experiments ± SD, **: p < 0.05.
3.5 Glucose uptake assays
Rat L6 skeletal muscle cells were used to study the effect of MB anthocyanins on glucose uptake in muscle cells. Rat L6 myoblasts were propagated and plated in 24-well plates and exposed to a differentiation program as described in Materials and Methods. No toxic effects were observed in any of the applied treatments. ANC produced a significant increase in the glucose uptake of muscle cells (Fig. 4A). Interestingly, and in agreement with our data obtained from the in-vivo glucose tolerance experiments (Fig. 2D), ANC significantly increased glucose uptake in L6 myotubes exposed to sub-optimal doses of insulin, suggesting an insulin-sensitising effect of ANC in muscle cells (Fig. 4B). No toxic effects of D3S5G were observed in muscle or liver cells.
Figure 4. Effect of MB anthocyanins on glucose uptake in L6 muscle cells.
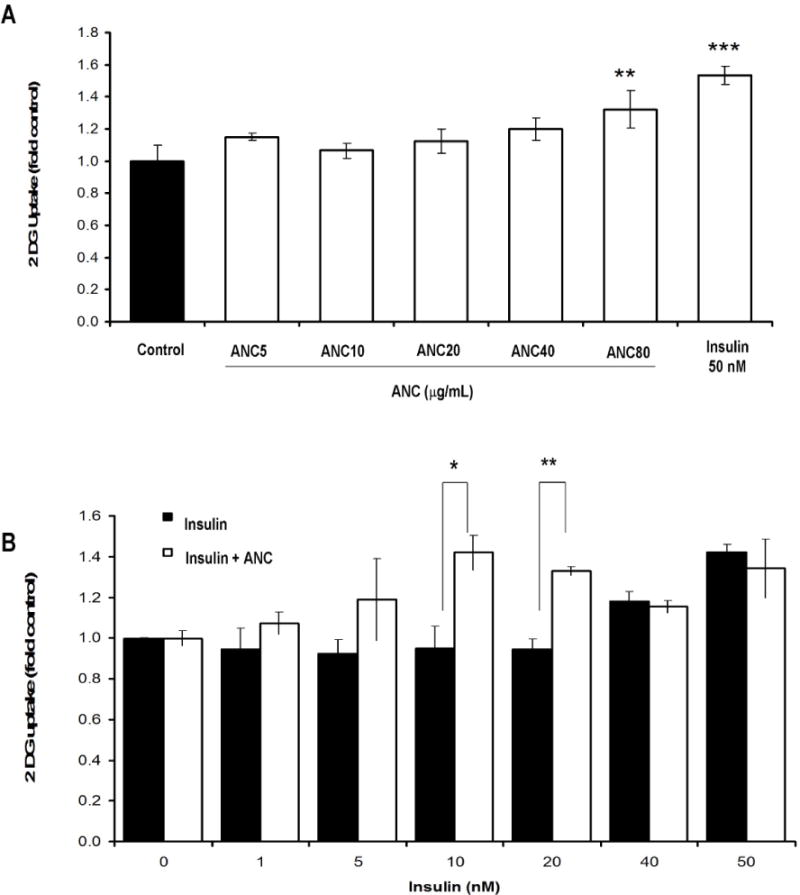
(A) Glucose uptake was tested in L6 muscle cells treated with a range of ANC concentrations (5–80 μg/ml) using no treatment as the negative control and insulin (50 nM) as the positive control. (B) Insulin sensitivity was assessed in L6 muscle cells using a range of insulin treatments (0–50 nM) either with or without ANC at 50μg/mL. Results are the mean three experiments +/− SD, *: p < 0.05, **: p <0.01, ***: p < 0.001.
3.6 Characterization and hypoglycaemic effects of D3S5G
Three formulations of MB extract were prepared, with increasing concentrations of total anthocyanins and designated as CE, PEA, and ANC (Table 1). The total concentration of anthocyanins in these preparations increased but the ratio of the individual anthocyanins remained constant during sequential purification steps. Delphinidin 3-sambubioside-5-glucoside (Fig. 5A), a distinctive anthocyanin of MB, was isolated from ANC and tested for hypoglycaemic activity using the same acute model that was used for the extract ANC (Fig. 5B). Even though delphinidin 3-glucoside (D3G) is one of the most abundant anthocyanins in MB, it was not a target in this study, since we recently reported that D3G isolated from blueberries lacks significant hypoglycaemic activity in this acute animal model of type II diabetes (Grace et al., 2009). D3S5G (Fig. 5A) is a characteristic anthocyanin of MB that is not reported in other common edible berries, such as blueberries (Grace et al., 2009), blackcurrant, açai, elderberry (Schreckinger et al., 2010) or cranberries (Milbury, Vita & Blumberg, 2010). FCPC, LC-MS and NMR were used to isolate, and structurally characterise D3S5G (Table 1, Fig. 1 and Fig. 5A). The chromatographic profile of the ANC and D3S5G is shown in Fig. 1. NMR analysis of the HPLC-purified D3S5G confirmed its identity and purity. Results showed that D3S5G was as effective as ANC, and comparable to metformin in normalising blood glucose levels of hyperglycaemic C57BL/6J mice (Fig. 5B). No cytotoxic effects of D3S5G were observed in muscle of liver cells. When tested directly in H4IIE cells, D3S5G significantly decreased glucose production in a dose-dependent manner (Fig. 5C) with no observable cytotoxic effects. Furthermore, D3S5G was able to inhibit glucose production in liver cells at lower doses than those of ANC (Figs. 4A and 5C). D3S5G caused a mild but significant increase in glucose uptake in muscle cells (Fig 5D).
Figure 5. Effect of D3S5G on glucose metabolism.
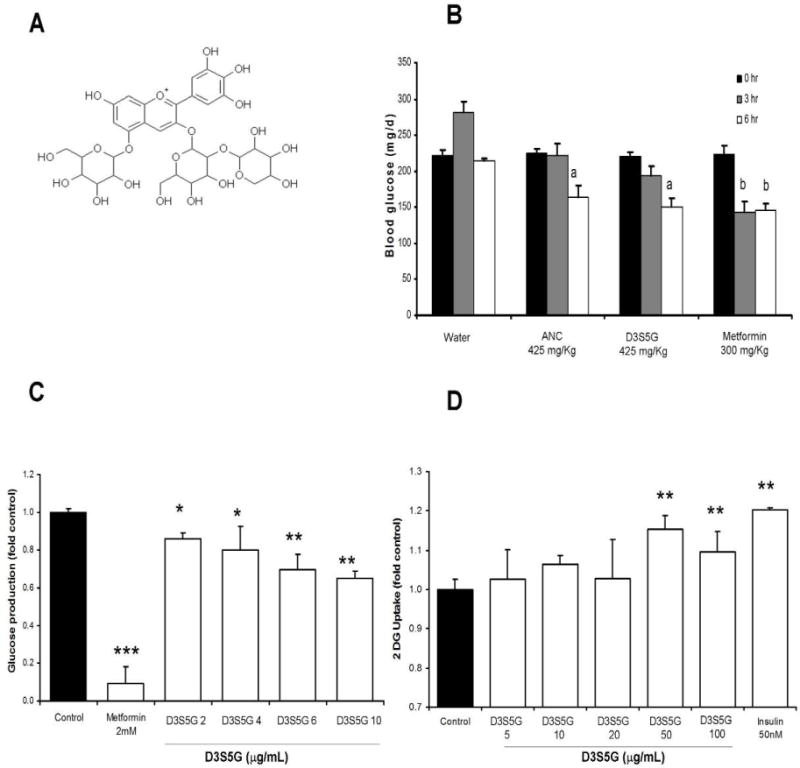
(A) Chemical structure of D3S5G. (B) Blood glucose levels of obese C57Bl6 hyperglycaemic obese mice were measured at 3 and 6 h after treatment with ANC and D3S5G (425 mg/kg BW) relative to metformin at 300 mg/kg. a: p < 0.01 vs time 0 h, b: p < 0.001 vs time 0 h. Values are mean of n = 6. (C) Glucose production was measured in H4IIE cells treated at doses of 2−10 μg/mL relative to metformin used at a dose of 2 mM and untreated controls. (D) Glucose uptake was measured in L6 muscle cells treated with D3S5G at doses of 5−100 μg/mL relative to insulin at 50 nM and untreated controls. Data in B and C are the mean +/− SD, *: p < 0.05 vs control, **: p < 0.01 vs control, ***: p < 0.001 vs control.
4. Discussion
Type II diabetes is by far the world’s most prevalent endocrine disorder which is characterised by chronic hyperglycaemia, alterations of lipid metabolism and dysfunctions of insulin response by peripheral tissues. Long term complications of type II diabetes are strongly correlated with chronic hyperglycaemia (Brown, Reynolds & Bruemmer, 2010); therefore, new methods to control hyperglycaemia are highly valuable. Botanicals in general and polyphenols in particular (Al-Awwadi et al., 2004a; Stull, Cash, Johnson, Champagne & Cefalu, 2010; Zunino, 2009) have been reported as potentially effective agents to control hyperglycaemia and insulin resistance (Ribnicky et al., 2009; Ribnicky, Poulev, Schmidt, Cefalu & Raskin, 2008; Ribnicky, Poulev, Watford, Cefalu & Raskin, 2006). Herein we report a novel standardised anthocyanin-rich formulation (ANC) from the edible fruit MB, which might help type II diabetic patients to prevent complications by controlling chronic hyperglycaemia. Our results provide complementary data to recent reports from in-vitro models of type II diabetes, which suggest that MB anthocyanins could benefit diabetic patients by inhibiting adipogenesis and inflammation (Schreckinger et al., 2010.). Also in line with Schreckinger’s work, another set of studies from Tsuda et al. demonstrated that cyanidin and cyaniding 3-glucoside ameliorate diabetes and insulin resistance by modulating lipid metabolism and energy expenditure (Tsuda et al., 2004; Tsuda, Ueno, Yoshikawa, Kojo & Osawa, 2006). The suggested mechanisms reported by Tsuda et al. are related to increased levels of adipocytokines (adiponectin and leptin) and the activation of AMP-activated protein kinase. In this context the present work provides new evidence on the potential role of anthocyanins from MB in controlling insulin resistance and diabetes by improving glucose metabolism in peripheral tissues. Our data show that oral administration of anthocyanins from MB reduces fasting hyperglycaemia in HFD obese diabetic mice and that D3S5G, an abundant and distinctive anthocyanin of MB, is at least partially responsible for the in-vivo anti-diabetic effects of MB anthocyanins (Fig. 2 and Fig. 5B). In addition ANC improved glucose tolerance in HFD obese hyperglycaemic mice (Fig. 2D), suggesting a potential insulin sensitisation by MB anthocyanins. This hypothesis was experimentally tested using insulin-stimulated glucose uptake in muscle cells and insulin-stimulated down regulation of G6P in liver cells, two distinct cellular models of type II diabetes. These results showed that ANC significantly increases insulin-stimulated glucose uptake in muscle cells (Fig. 4B) and enhances the insulin-stimulated downregulation of gluconeogenic enzyme G6P in liver cells (Fig. 3C). Thus, the improvement in glucose tolerance observed in HDF mice treated with ANC may result from an improved insulin-mediated glucose metabolism in skeletal muscle and liver, although other physiological mechanisms cannot be ruled out. Both ANC and D3S5G decreased glucose production in liver cells (Figs. 3A and 5C) and ANC caused a mild decrease in the mRNA levels of the G6P enzyme (Fig. 3B) but D3S5G did not affect G6P mRNA levels (unpublished observation), thus suggesting that other components of the extract are contributing to this antidiabetic effect. D3S5G only represents 6.76% of the ANC extract, while other anthocyanins constitute 54.67 % (see Table 1), together with other polyphenolic compounds like flavonoids. Those polyphenolic compounds might work individually or synergistically to potentiate the antidiabetic activity of the ANC extract. Similarly, anthocyanin enriched fractions of bilberry have been reported to produce hypoglycaemic and insulin sensitising effects in type II diabetic mice (Takikawa, Inoue, Horio & Tsuda, 2010). Although, these authors did not report the antidiabetic activity of any pure anthocyanin from bilberry, they found that conjugated forms of delphinidin account for up to 38.7% of total anthocyanins of the active fraction of bilberry. This suggests that conjugated forms of delphinidin may be relevant to the antidiabetic properties of bilberry. Delphinidin 3-glucoside (D3S) does not display significant hypoglycaemic effect in HFD diabetic mice (Grace et al., 2009) and in the present work we found that another delphinidin-derived anthocyanin, D3S5G, displays potent in-vivo and in-vitro antidiabetic properties. Therefore, even though D3S5G may not be the only active compound in MB, it is seemingly a good candidate for a marker compound of antidiabetic formulations obtained from MB. Earlier, we were using Labrasol, a chemical bioenhancing/solubilising excipient, as a means to increase the oral anti-diabetic effects of anthocyanin-rich formulations (Grace et al., 2009). We used this approach in the present work and observed that the hypoglycaemic effects of ANC did not require the co-administration of Labrasol to reduce fasting blood glucose levels.(Fig. 2B). This apparent advantage of MB anthocyanins over those from blueberry (Grace et al., 2009) might result from an improved bioavailability of MB anthocyanins derived from different combinations of specific aglycones and sugar conjugation patterns. Further investigations are needed to elucidate the specific absorption and/or elimination mechanisms of maqui anthocyanins and also their interactions with the intestinal microflora.
Conclusions
ANC is an anthocyanin-rich formulation from MB which improves hyperglycaemia and insulin resistance in obese hyperglycaemic mice fed a high fat diet. ANC seems to function by modulating glucose metabolism in the skeletal muscle and liver. To our knowledge, this is the first report describing the anti-diabetic properties and potential mode of action of MB anthocyanins. One of MB’s distinctive anthocyanins, D3S5G, displays insulin-like effects in muscle and liver cells and seems to be partially responsible for the anti-diabetic effect of ANC. As MB’s anthocyanins are regularly consumed as fresh fruits or juice concentrates, we propose ANC as a novel natural source of potentially safe anti-diabetic compounds. Further investigations into the effects of ANC on the glucose metabolism of type II diabetes patients will need to be completed for the most effective use of ANC for the treatment and prevention of the disease.
Highlights.
Anthocyanins (ANC) from Aristotelia chilensis improve fasting blood glucose levels and glucose tolerance in obese diabetic mice.
ANC decreases glucose production and enhances insulin-induced gene expression of glucose-6-phosphatase in liver cells.
ANC increases both insulin and non-insulin mediated glucose uptake in L6 myotubes.
Delphinidin 3-sambubioside-5-glucoside (D3S5G) is isolated and identified as a characteristic anthocyanin from maqui berry.
D3S5G decreases fasting hyperglycaemia in obese diabetic mice and improves glucose metabolism in liver and muscle cells.
Acknowledgments
This work supported in part by the by Fundación Chile, Santiago, Chile and by the Botanical Research Center grant P50 AT002776 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS) which funds the Botanical Research Center of Pennington Biomedical Research Center, Louisiana State University and Rutgers University.
Abbreviations
- ANC
Anthocyanin Rich Fraction of Maqui Berry
- D3G5G
Delphinidin-3,5-diglucoside
- D3S5G
Delphinidin-3-sambubioside-5-glucoside
- C3S5G
Cyanidin-3-sambubioside-5-glucoside
- C3G5G
Cyanidin-3,5-diglucoside
- C3G
Cyanidin-3-glucoside
- C3S
Cyanidin-3-sambubioside
- D3S
Delphinidin-3-sambubioside
- D3G
Delphinidin-3-glucoside
- MB
Maqui Berry
- PAE
Post Amberlite Extract of Maqui Berry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Awwadi N, Azay J, Poucheret P, Cassanas G, Krosniak M, Auger C, Gasc F, Rouanet JM, Cros G, Teissedre PL. Antidiabetic activity of red wine polyphenolic extract, ethanol, or both in streptozotocin-treated rats. J Agric Food Chem. 2004a;52(4):1008–1016. doi: 10.1021/jf030417z. [DOI] [PubMed] [Google Scholar]
- Al-Awwadi NA, Bornet A, Azay J, Araiz C, Delbosc S, Cristol JP, Linck N, Cros G, Teissedre PL. Red wine polyphenols alone or in association with ethanol prevent hypertension, cardiac hypertrophy, and production of reactive oxygen species in the insulin-resistant fructose-fed rat. J Agric Food Chem. 2004b;52(18):5593–5597. doi: 10.1021/jf049295g. [DOI] [PubMed] [Google Scholar]
- Araya H, Clavijo C, Herrera C. Antioxidant capacity of fruits and vegetables cultivated in Chile. Arch Latinoam Nutr. 2006;56(4):361–365. [PubMed] [Google Scholar]
- Brown A, Reynolds LR, Bruemmer D. Intensive glycemic control and cardiovascular disease: an update. Nat Rev Cardiol. 2010;7(7):369–375. doi: 10.1038/nrcardio.2010.35. [DOI] [PubMed] [Google Scholar]
- Cespedes C, El-Hafidi M, Pavon N, J A. Antioxidant and cardioprotective activities of pheniloic extracts from fruits of Chilean blackberry Aristotelia chilenesis (Elaeocarpaceae), Maqui. Food Chemistry. 2008;108:820–829. [Google Scholar]
- Cespedes C, J A, Avila J, Nieto J. Anti-inflammatory Activity of Aristotelia chilensis Mol. (Stuntz) (Elaeocarpaceae) Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas. 2010;9(27):127–135. [Google Scholar]
- Céspedes C, Valdez-Morales M, Avila J, El-Hafidi M, Alarcón J, Paredes-López O. Phytochemical profile and the antioxidant activity of Chilean wild black-berry fruits, Aristotelia chilensis (Mol) Stuntz (Elaeocarpaceae) Food Chemistry. 2010;1(19):886–895. [Google Scholar]
- Cespedes CL, Alarcon J, Valdez-Morales M, Paredes-Lopez O. Antioxidant activity of an unusual 3-hydroxyindole derivative isolated from fruits of Aristotelia chilensis (Molina) Stuntz. Z Naturforsch C. 2009;64(9–10):759–762. doi: 10.1515/znc-2009-9-1024. [DOI] [PubMed] [Google Scholar]
- Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26(8):1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- de Raemy-Schenk AM, Trouble S, Gaillard P, Page P, Gotteland JP, Scheer A, Lang P, Yeow K. A cellular assay for measuring the modulation of glucose production in H4IIE cells. Assay Drug Dev Technol. 2006;4(5):525–533. doi: 10.1089/adt.2006.4.525. [DOI] [PubMed] [Google Scholar]
- Degenhardt A, Knapp H, Winterhalter P. Separation and purification of anthocyanins by high-speed countercurrent chromatography and screening for antioxidant activity. J Agric Food Chem. 2000;48(2):338–343. doi: 10.1021/jf990876t. [DOI] [PubMed] [Google Scholar]
- Escribano-Bailon MT, Alcalde-Eon C, Munoz O, Rivas-Gonzalo JC, Santos-Buelga C. Anthocyanins in berries of Maqui (Aristotelia chilensis (Mol.) Stuntz) Phytochem Anal. 2006;17(1):8–14. doi: 10.1002/pca.872. [DOI] [PubMed] [Google Scholar]
- Grace MH, Ribnicky DM, Kuhn P, Poulev A, Logendra S, Yousef GG, Raskin I, Lila MA. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine. 2009;16(5):406–415. doi: 10.1016/j.phymed.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman A. Flora silvestre de Chile zona araucana. Santiago: Fundacion Claudio Gay; 1991. [Google Scholar]
- Kizelsztein P, Govorko D, Komarnytsky S, Evans A, Wang Z, Cefalu WT, Raskin I. 20-Hydroxyecdysone decreases weight and hyperglycemia in a diet-induced obesity mice model. Am J Physiol Endocrinol Metab. 2009;296(3):E433–439. doi: 10.1152/ajpendo.90772.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Thomas TC, Storlien LH, Huang XF. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord. 2000;24(5):639–646. doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- Loike JD, Zalutsky DL, Kaback E, Miranda AF, Silverstein SC. Extracellular creatine regulates creatine transport in rat and human muscle cells. Proc Natl Acad Sci U S A. 1988;85(3):807–811. doi: 10.1073/pnas.85.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbury PE, Vita JA, Blumberg JB. Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J Nutr. 2010;140(6):1099–1104. doi: 10.3945/jn.109.117168. [DOI] [PubMed] [Google Scholar]
- Miranda-Rottmann S, Aspillaga AA, Perez DD, Vasquez L, Martinez AL, Leighton F. Juice and phenolic fractions of the berry Aristotelia chilensis inhibit LDL oxidation in vitro and protect human endothelial cells against oxidative stress. J Agric Food Chem. 2002;50(26):7542–7547. doi: 10.1021/jf025797n. [DOI] [PubMed] [Google Scholar]
- Morariou M. U P Office. A1. 2007/0065396. United States: Tracie Martyn International, LLC; 2007. Topical Macqui Berry Formularion; pp. 1–22. [Google Scholar]
- Munoz M, Barrera E, Meza I. EL uso Medicinal y Alimenticio de las Plantas Nativas y Naturalizadas en Chile. Santiago, Chile: 1981. [Google Scholar]
- Ribnicky DM, Kuhn P, Poulev A, Logendra S, Zuberi A, Cefalu WT, Raskin I. Improved absorption and bioactivity of active compounds from an anti-diabetic extract of Artemisia dracunculus L. Int J Pharm. 2009;370(1–2):87–92. doi: 10.1016/j.ijpharm.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribnicky DM, Poulev A, Schmidt B, Cefalu WT, Raskin I. Evaluation of botanicals for improving human health. Am J Clin Nutr. 2008;87(2):472S–475S. doi: 10.1093/ajcn/87.2.472S. [DOI] [PubMed] [Google Scholar]
- Ribnicky DM, Poulev A, Watford M, Cefalu WT, Raskin I. Antihyperglycemic activity of Tarralin, an ethanolic extract of Artemisia dracunculus L. Phytomedicine. 2006;13(8):550–557. doi: 10.1016/j.phymed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Hermosin-Gutierrez I, Mardones C, Vergara C, Herlitz E, Vega M, Dorau C, Winterhalter P, von Baer D. Polyphenols and antioxidant activity of calafate (Berberis microphylla) fruits and other native berries from Southern Chile. J Agric Food Chem. 2005;58(10):6081–6089. doi: 10.1021/jf100173x. [DOI] [PubMed] [Google Scholar]
- Schreckinger M, Wang J, Yousef G, Lila M, de Mejia E. Antioxidant capacity and in vitro inhibition of adipogenesis and inflammation by phenolic extracts of Vaccinium floribundum and Aristotelia chilensis. J Agric Food Chem. 2010;58:8966–8976. doi: 10.1021/jf100975m. [DOI] [PubMed] [Google Scholar]
- Schreckinger ME, Lotton J, Lila MA, de Mejia EG. Berries from South America: a comprehensive review on chemistry, health potential, and commercialization. J Med Food. 2010;13(2):233–246. doi: 10.1089/jmf.2009.0233. [DOI] [PubMed] [Google Scholar]
- Stull AJ, Cash KC, Johnson WD, Champagne CM, Cefalu WT. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr. 2010;140(10):1764–1768. doi: 10.3945/jn.110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37(9):1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- Takikawa M, Inoue S, Horio F, Tsuda T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J Nutr. 2010;140(3):527–533. doi: 10.3945/jn.109.118216. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Horio F, Uchida K, Aoki H, Osawa T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr. 2003;133(7):2125–2130. doi: 10.1093/jn/133.7.2125. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Ueno Y, Aoki H, Koda T, Horio F, Takahashi N, Kawada T, Osawa T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem Biophys Res Commun. 2004;316(1):149–157. doi: 10.1016/j.bbrc.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Ueno Y, Yoshikawa T, Kojo H, Osawa T. Microarray profiling of gene expression in human adipocytes in response to anthocyanins. Biochem Pharmacol. 2006;71(8):1184–1197. doi: 10.1016/j.bcp.2005.12.042. [DOI] [PubMed] [Google Scholar]
- Wilson T, Meyers SL, Singh AP, Limburg PJ, Vorsa N. Favorable glycemic response of type 2 diabetics to low-calorie cranberry juice. J Food Sci. 2008;73(9):H241–245. doi: 10.1111/j.1750-3841.2008.00964.x. [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Asilmaz E, Luca E, Friedman JM, Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432(7020):1027–1032. doi: 10.1038/nature03047. [DOI] [PubMed] [Google Scholar]
- Zunino S. Type 2 diabetes and glycemic response to grapes or grape products. J Nutr. 2009;139(9):1794S–1800S. doi: 10.3945/jn.109.107631. [DOI] [PubMed] [Google Scholar]


