Figure 5. Effects of test compounds on reactivation of quiescent HSV-1 infection in iPSC-neurons.
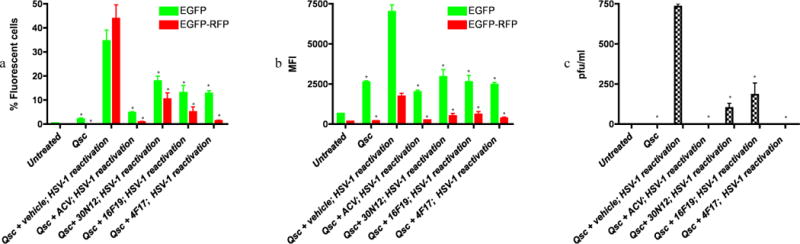
FC was performed after reactivation of quiescently infected iPSC-neuronal cultures 48h as described. (a) percentage of fluorescent cells. *p≤0.027. (b) mean fluorescence intensity (MFI). *p≤0.016. (c) Plaque assays of virus produced in the supernatant of reactivated cells titrated on Vero cells, shown as plaque forming units per milliliter (PFU/ml). *p≤0.002. The data represent an average of three independent experiments. 5BVdU: (E)-5-(2-bromovinyl)-2′-deoxyuridine, IFN-α: interferon alpha, NaB: sodium butyrate, vehicle: DMSO. Error bars represent standard deviations.
“Qsc”: Quiescent HSV-1 infection established by infecting iPSC-neurons with HSV-1 and then culturing in neurobasal medium containing 5BVdU+IFN-α for 7 days (See methods).
“Qsc+vehicle or compounds (ACV, 4F17, 16F19, or 30N12); HSV-1 reactivation”: 5BVdU+IFN-α were withdrawn and cells were cultured in the presence of vehicle or compounds for 48h; HSV-1 was then reactivated by treatment with NaB for 5 days.
