Table 4.
Probing N4-substituents of N2-furfuryl-quinazolines
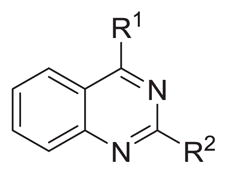
| |||||
|---|---|---|---|---|---|
| Compound | R1 | R2 |
L. donovani EC50a (μM) |
J774A.1 EC50b (μM) |
SI |
| 3 |
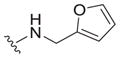
|
|
2.5 ± 0.4c | 17 ± 6c | 6.8 |
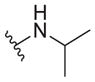
| 28 |
|
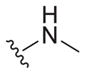
|
>25 | ND | ND |
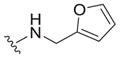
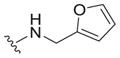
| 29 |
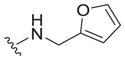
|
|
>25 | ND | ND |
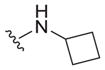
| 30 |
|
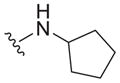
|
>25 | ND | ND |
| 31 |
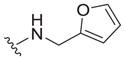
|
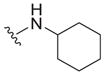
|
4.0 ± 2.5 | 5.3 ± 0.8 | 1.3 |
EC50 value is the mean ± standard deviation of at least three independent experiments. When a mean value could not be ascertained, the reported value is based on at least two determinations. The control drug for the in vitro intracellular antileishmanial assay is amphotericin B, which displays an EC50 = 41 ± 8 nM against L. donovani (n = 20).
EC50 value is the mean ± standard deviation of at least three independent experiments. Podophyllotoxin is the control compound for the in vitro cytotoxicity assay, exhibiting an EC50 = 21 ± 5 nM against the J774.A1 macrophages (n = 11).
From Van Horn et al.18
