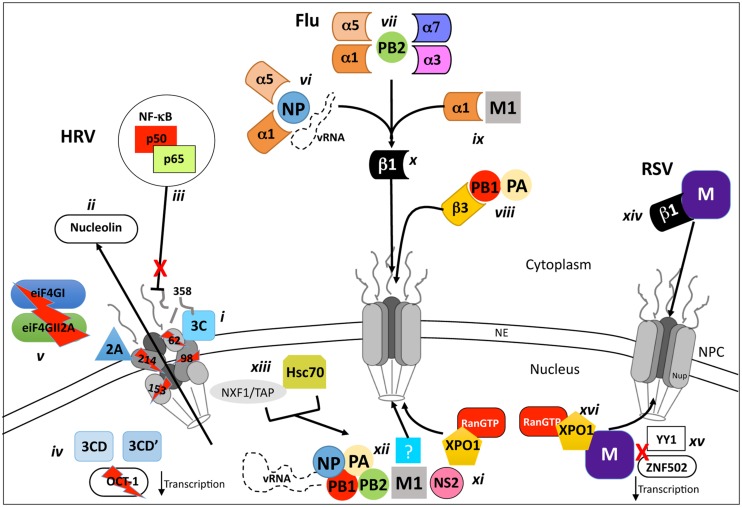
FIGURE 2.
Schematic representation of VRD modulation and/or exploitation of host nucleocytoplasmic transport processes. Inhibition and/or utilization of host-cell nucleocytoplasmic transport are key features of infection by Rhinovirus (HRV), Influenza virus and RSV. During HRV infection, the viral proteases 2A and 3C localize to the NPC (i) and degrade nups 62, 98, 153, 214, and 358, causing mislocalization of nuclear proteins such as nucleolin (ii) and preventing nuclear import of complexes such as the anti-viral NF-κB transcription factor (iii). Host-cell transcription/translation is severely reduced by the NLS of the 3CD and 3CD’ proteases which degrade the general transcription factor OCT-1 (iv) in concert with 2A, which also degrades the cytoplasmic translation elongation factors eIF4GI and eIF4GII2A (v). Efficient influenza virus replication requires the transport of the viral genome and proteins required for its replication (PB1, PB2, PA, and NP) to the nucleus where they form a vRNP complex. The vRNA is transported to the nucleus through binding to NP, which is recognized by IMPα1 or α5 (vi) in complex with Impβ1 (x), is transported through the NPC, as is PB2 (vii), which is recognized by either IMPα7, α5, α3, or α1 in complex with IMPβ1 (x). The PB1/PA heterodimer is transported to the nucleus by interaction with the IMPβ1 homologue IPO5 (viii) which can bind the NPC directly. The M1 protein, critical for the nuclear export of the vRNP complex is imported to the nucleus via IMPα1/β1 (ix,x). The newly synthesized vRNA (part of the vRNP-N1-NS2 complex) is exported from the nucleus by XPO1 interaction with NS2 (xi). An unknown exporter (xii) that interacts with M1 has been implicated in this process, as have the proteins Hsc70 and NXF1/TAP (xiii), which are postulated to act as cofactors via an undefined mechanism. The RSV M protein relies on interaction with Impβ1 (xiv) early during infection to localize to the nucleus where it suppresses host-cell transcription by potentially blocking the activity of transcription factors such as ZNF502 and YY1 (xv). M is exported to the cytoplasm later in infection by XPO1 (xvi), where it is critical for pro-virion assembly.