FIGURE 8.
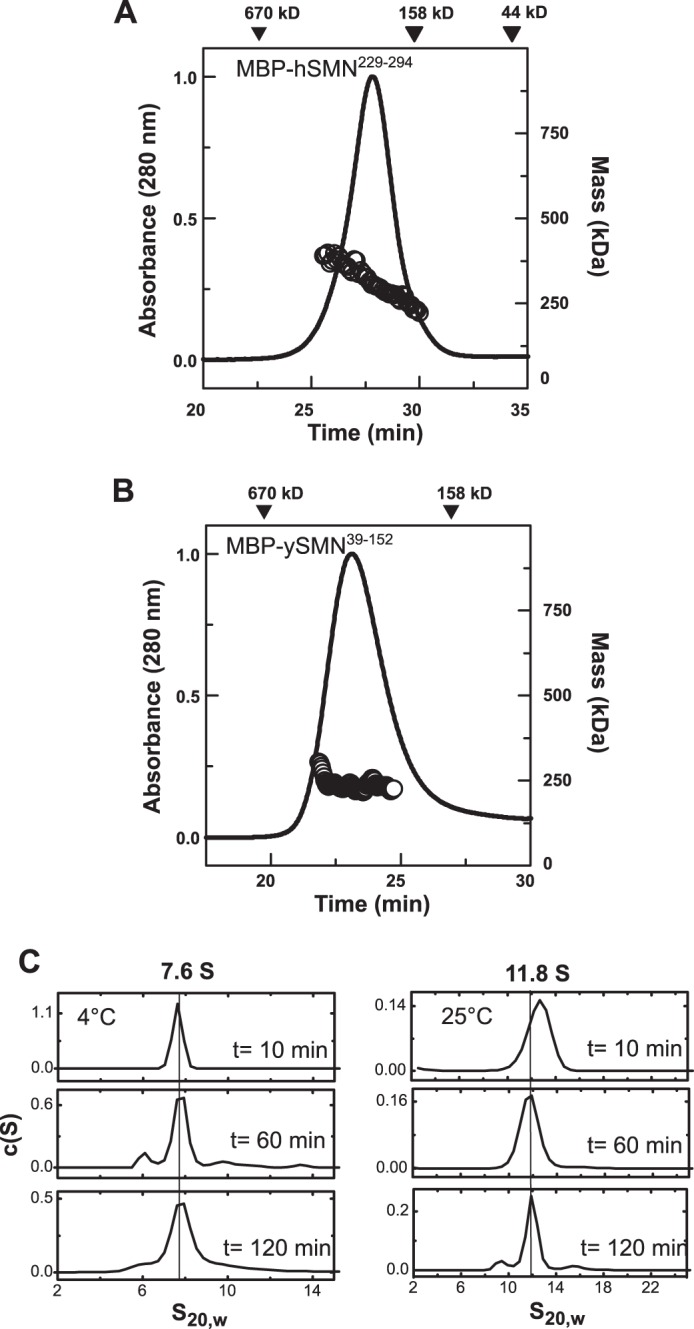
Oligomeric properties of MBP-SMN fusions. A, SEC-MALS analysis of MBP-hSMN(229–294) at 20 °C. The MBP-SMN fusion (48 kDa) forms oligomers with Mw = 200–400 kDa, spanning the tetramer to octamer range. B, SEC-MALS analysis of MBP-ySMN(39–152) at 20 °C. The 54-kDa fusion protein has an average Mw of 189 kDa across the eluted peak. C, SV analyses of MBP-hSMN(229–294) at 4 °C and 25 °C. Like hSMN·Gemin2, the MBP-hSMN fusion displays a temperature-dependent oligomerization. The larger 11–12 S oligomers observed at 25 °C are destabilized at 4 °C, where a 7.6 S species is observed.
