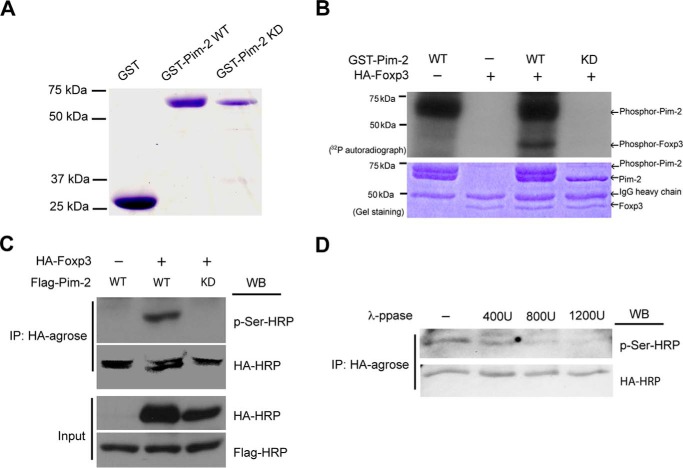
FIGURE 2.
Foxp3 was phosphorylated by Pim-2 in vitro and in vivo. A, recombinant GST, GST-Pim-2 WT, and GST-Pim-2 KD were expressed in E. coli cells and purified with glutathione-Sepharose beads. B, the kinase assay was analyzed with 32P incorporation. The agarose-bound HA-Foxp3 was immunoprecipitated from the cell lysates of Foxp3-transfected HEK293T cells and used as substrate for the kinase assay in vitro. Experiment details are described under “Experimental Procedures.” C, phosphorylation of Foxp3 by Pim-2 in transfected HEK293T cells was detected by Western blot (WB) analysis. D, phosphorylation of Foxp3 by Pim-2 can be reversed by λ-phosphatase in vitro. The agarose-bound HA-Foxp3 was immunoprecipitated from Foxp3- and Pim-2-cotransfected 293T cell lysates and then incubated with different concentrations of λ-phosphatase as indicated. The reactions were carried out at 30 °C for 30 min, terminated by adding 2× loading buffer, and then subjected to 8% SDS-PAGE and Western blot analysis.