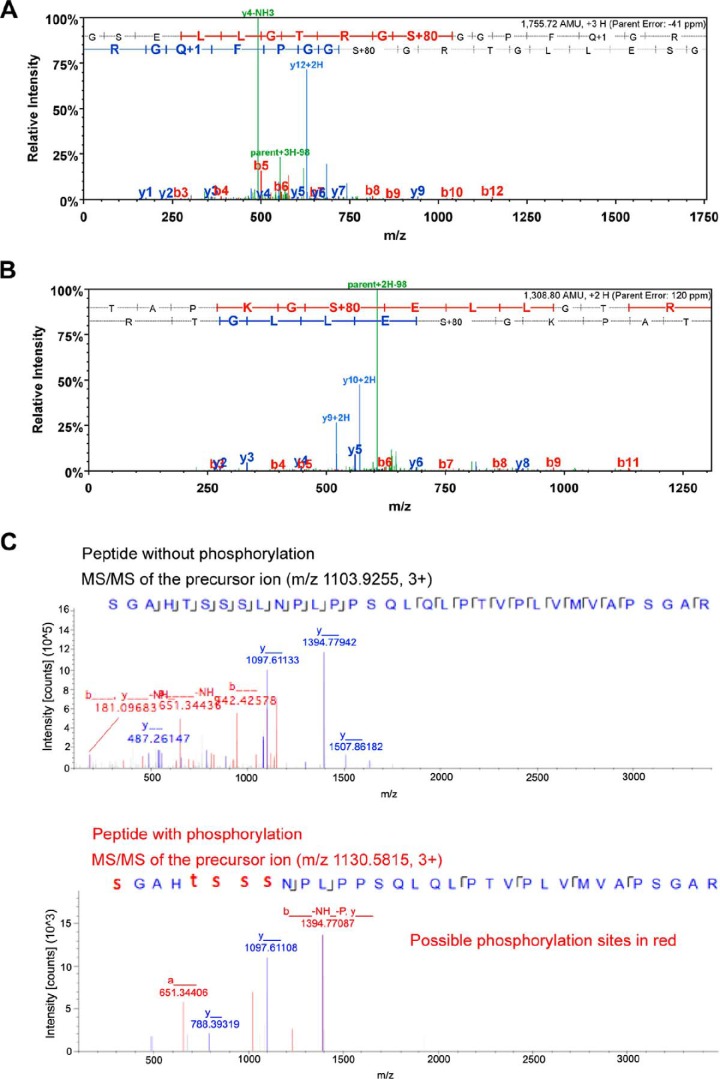
FIGURE 3.
MS/MS analysis identified three phosphorylated peptides of mouse Foxp3. Foxp3 protein was purified from Pim-2- and Foxp3-cotransfected 293T cells. A, MS/MS spectrum of the phosphorylated peptide, 28TAPKGS*ELLGTRG40. S* indicates that serine residue 33 is phosphorylated by Pim-2. B, MS/MS spectrum of the phosphorylated peptide, 32GSELLGTRGS*GGPFQGRD49. S* indicates that serine residue 41 is phosphorylated by Pim-2. C, comparison of the MS/MS spectra of m/z 1103.9255 (unphosphorylated) and m/z 1130.5815 (phosphorylated) peptide, 52S*GAHT*S*S*S*LNPLPPSQLQLPTVPLVMVAPSGAR84. S* and T* suggest the phosphorylation site to be either serine or threonine among 52, 56, 57, 58, or 59 residues. In the phosphorylation assignment (panel A spectrum), the phosphorylation site could be at either Thr-28, Ser-33, or Thr-38 in this peptide sequence. Nevertheless, the observation of the y11/y12 ions eliminated the Thr-28 possibility (could only at either Ser-33 or Thr-38 sites). Furthermore, the observation of the y4 ion eliminated the Thr-38 possibility, and the observation of y9 ion further confirmed the phosphorylation at the Ser-33 site. The same approach of using key MS2 spectrum ions was conducted for the phosphorylation site assignments for panels B and C.