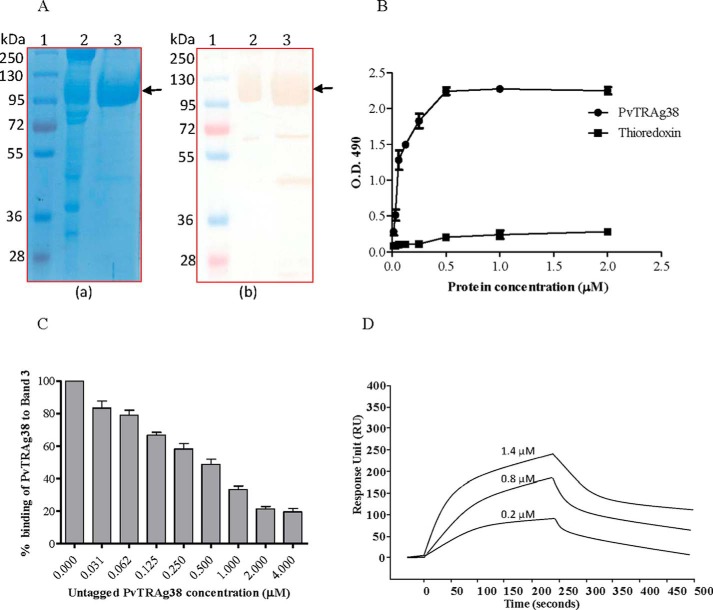
FIGURE 6.
Binding of PvTRAg38 with purified Band 3 protein. A, Band 3 was purified from human erythrocytes using the protocol of Casey et al. (22) by affinity chromatography on aminoethyl-agarose resin. RBC membrane preparations and purified Band 3 were analyzed by SDS-PAGE (panel a) followed by Western blot analysis using anti-Band 3 monoclonal antibodies (panel b). Lane 1, molecular weight marker; lane 2, RBC membrane; lane 3, purified Band 3. The monoclonal anti-Band 3 antibody reacted with full-length Band 3, as well as with truncated Band 3. Arrows indicate the position of Band 3. B, binding of recombinant PvTRAg38 to the purified Band 3 by solid phase ELISA. Increasing concentrations (0–2 μm) of histidine-tagged PvTRAg38 or bacterial thioredoxin from D. desulfuricans (negative control) were added to the wells of an ELISA plate already coated with 50 nm of Band 3. The plate was developed with mouse anti-His6 monoclonal antibody as described in the text. The mean ± S.D. value of absorbance from three experiments is plotted. C, specificity of binding of PvTRAg38 to Band 3 by competition assay. Increasing concentrations of untagged PvTRAg38 was mixed with fixed concentration (0. 5 μm) of histidine-tagged PvTRAg38 and allowed to interact with 50 nm of immobilized Band 3 for 4 h at 37 °C. Bound recombinant histidine-tagged PvTRAg 38 was detected with mouse anti-His6 monoclonal antibody as described in the text. Binding in the absence of untagged PvTRAg38 was taken as percentage control for the rest of the concentrations. The mean value of three independent experiments is plotted with S.D. D, SPR analysis of Band 3 interaction with PvTRAg38. Band 3 was immobilized on the cell of CM5 chip. Three different concentrations of PvTRAg38 (0.2, 0.8, and 1.4 μm) were injected at flow rate of 30 μl/min over the surface of immobilized Band 3. Sensogram curves show dose-dependent response of PvTRAg38 binding with Band 3.