Background: Upon entering the pancreatic β-cell, glucose is metabolized to ultimately induce both proliferation and the release of insulin.
Results: miR-184 targets Argonaute2 to impact the microRNA pathway according to glucose metabolism.
Conclusion: miR-184 is a highly regulated microRNA impacting the growth and function of the β-cell.
Significance: These results highlight the adaptive role of the microRNA pathway based on metabolic state.
Keywords: Argonaute, beta cell (B-cell), glucose metabolism, insulin, insulin secretion, microRNA (miRNA), microRNA mechanism, pancreatic islet
Abstract
In response to fasting or hyperglycemia, the pancreatic β-cell alters its output of secreted insulin; however, the pathways governing this adaptive response are not entirely established. Although the precise role of microRNAs (miRNAs) is also unclear, a recurring theme emphasizes their function in cellular stress responses. We recently showed that miR-184, an abundant miRNA in the β-cell, regulates compensatory proliferation and secretion during insulin resistance. Consistent with previous studies showing miR-184 suppresses insulin release, expression of this miRNA was increased in islets after fasting, demonstrating an active role in the β-cell as glucose levels lower and the insulin demand ceases. Additionally, miR-184 was negatively regulated upon the administration of a sucrose-rich diet in Drosophila, demonstrating strong conservation of this pathway through evolution. Furthermore, miR-184 and its target Argonaute2 remained inversely correlated as concentrations of extracellular glucose increased, underlining a functional relationship between this miRNA and its targets. Lastly, restoration of Argonaute2 in the presence of miR-184 rescued suppression of miR-375-targeted genes, suggesting these genes act in a coordinated manner during changes in the metabolic context. Together, these results highlight the adaptive role of miR-184 according to glucose metabolism and suggest the regulatory role of this miRNA in energy homeostasis is highly conserved.
Introduction
Our understanding of the compensatory mechanisms controlling pancreatic β-cell function according to changes in extracellular glucose remains incomplete (1, 2). Upon entering the β-cell, glucose is metabolized to ultimately induce both proliferation and the release of insulin (3–5). However, the full extent to which genes that regulate growth and secretion respond to the molecular events that follow glycolysis, including mitochondrial and ATP metabolism, is not completely known. Recent studies have shown that deletion of glucokinase (Gck) reduced β-cell replication, underlining the role of glucose as a key mitogenic factor in this cell type (6). In addition, the BCL-2 family member BAD occupies a glucokinase-containing complex to regulate mitochondrial respiration in the β-cell in response to glucose (7). Moreover, in response to a glucose stimulus, quiescent β-cells entered the G1 phase of the cell cycle further emphasizing the tight association between the glycolytic pathway and the mechanisms regulating β-cell mass (8). Specific cell cycle regulators and polycomb group (PcG) proteins have also been shown to regulate β-cell proliferation and regeneration, indicating that many conserved and functionally diverse factors contribute to this process (9, 10). Furthermore, the growth rate of the β-cell declines with age in both mouse and human, indicating many regulatory mechanisms act in an age-dependent manner (11, 12).
We recently showed that miR-184, a highly conserved and abundant miRNA2 expressed in the β-cell, regulates compensatory proliferation and insulin secretion during insulin resistance (13). Using both genetic and diet-induced mouse models of obesity and insulin resistance, we observed the silencing of miR-184 in the pancreatic islet, and upon treatment of a low carbohydrate ketogenic diet, its expression was rescued (13). Importantly, these results suggested that miRNAs may respond to changes in the metabolic environment of the cell including systemic insulin sensitivity and glucose concentrations. Although the precise role of the miRNA pathway remains to be established, many studies have highlighted its regulatory role in gene regulation during adaptive response mechanisms (14). Under steady-state conditions, many loss-of-function mouse models for miRNA genes exhibit subtle phenotypes that become more pronounced upon the induction of physiologic stresses (15). To date, the impact of changes in nutrient intake and sensing on the miRNA pathway has not been characterized. Therefore, our main goal was to identify the extent to which miRNAs are altered according to extracellular glucose levels and to determine the functional relevance of their regulation.
In this study we first reinvestigated the impact of a long term ketogenic diet on miR-184 expression in the pancreatic β-cell; as in our previous work we found that administration of this diet to hyperglycemic ob/ob mice restored both insulin sensitivity and normoglycemia (13). Furthermore, our studies also show that reverting from a ketogenic diet back to a normal chow restores miR-184 expression to normal within 24 h, illustrating the modulatory behavior of this one specific miRNA. Moreover, fasting and inhibition of glycolysis both resulted in increased levels of miR-184, indicating this miRNA is activated to suppress secretion as the demand for insulin is abated. Lastly, our observations showing the silencing of miR-184 in response to a high sucrose diet in Drosophila suggests this miRNA may contribute to a highly conserved mechanism regulating energy homeostasis. Together these results identify the adaptive functional role of miR-184 according to glucose metabolism and establish the conservation of its modulatory behavior to Drosophila.
Experimental Procedures
Generation and Maintenance of Animals
Mice were maintained on a 12-h light/dark cycle with ad libitum access to regular chow food or ketogenic diet (catalog number E15149-30, ssniff Spezialdiäten GmbH) in accordance with requirements established by Landesamt für Gesundheit und Soziales (Lageso). All experimental procedures were approved under protocols G 0357/10, O 0405/09, and T 0436/08. The total miR-184 knock-out (184KO), dox-184, and dox-Ago2 mice were generated and genotyped as previously described (13).
Gene Expression Array Analysis
MIN6 cells were transfected with rtTA reverse transactivator along with 184-tetO plasmids. Overexpression of miR-184 was induced by 1 μg/ml doxycycline (Sigma) at time points between 16 and 72 h in triplicate. Cells were harvested, and cDNA synthesis was performed from total RNA using the Illumina TotalPrep RNA Amplification kit (AMIL1791, Life Technologies) and then hybridized using Illumina mouse WG6v2 arrays. Raw data from the Illumina scanner were loaded into R using the lumi package (Illumina). Mappings to gene names and gene IDs were provided by the lumiMouseIDMapping package. Light intensities were quantile-normalized using the lumiN function, and the analysis focused on probes for the detection of p values <0.05 either in the transfection control or at any of the time points of the experiment. For subsequent analyses, we focused on these probes, discarding all others. Mappings of probes to gene IDs were obtained from the lumiMouseAll.db package, and we computed the differential regulation in gene expression as the log 2-fold change in signal intensity at the different time points compared with the transfection control. We investigated the effect of the miR-184 induction on the miR-184 target genes as well as on the target genes of miR-375, miR-182, miR-30a, and 148a/152 that are highly expressed in MIN6 cells. For each of these four miRNAs, we collected groups of target genes according to the presence of a canonical binding site in the 3′-UTR, defined as a heptamer complementary to positions 2–8 of the miRNA, or to positions 2–7 with a 'U' at position 1 (16). A fifth group (which we called “no seed”) consisted of genes with no canonical binding site for any of these miRNAs in the 3′-UTR. 3′-UTR sequences were downloaded from the RefSeq database (NCBI) on January 18, 2011. For each of these groups of genes and for each time point, we finally computed the mean log 2-fold change in gene expression upon miR-184 induction as well as the standard error.
Gene Expression Analysis in Drosophila
Canton-s flies were maintained at 25 °C in 12 h light:12 h dark cycles on a standard diet (yeast, 38 g/liter; yellow corn mill, 91 g/liter; agar, 10 g/liter; molasses, 8.7% v/v; propanoic acid (BioLab), 0.9% v/v; Tegasept solution (Sigma; 300 g/liter in EtOH (BioLab)), 0.8% v/v). For experimental manipulation, 3-day-old canton-s flies were starved for 16 h and then supplemented with sucrose (2% agar and 5% sucrose food media), and flies were collected at 0, 6, 12, and 18 h. RNA was generated from fly heads by using TRIzol reagent (Invitrogen). RNA was incubated with poly(A) polymerase (Ambion), and cDNA was synthesized using oligo-dT primers. qRT-PCR was performed with Bio-Rad (C 1000TM Thermal cycler) real time PCR. The following primer sequences were used in gene study: mir-184 GACGGAGAACTGATAAGGG; ribosomal protein 49 (rp-49), CGGTTACGGATCGAACA; universal primer, GCGAGCACAGAATTAATACGAC. miRNA values were normalized with the ribosomal protein 49 (rp-49).
Cell Culture, Biochemical Fractionation, and Antibodies
MIN6 cells were cultured in DMEM (Invitrogen) containing 4.5 g/liter glucose supplemented with 15% v/v heat-inactivated FCS, 50 μm β-mercaptoethanol, and 50 mg/ml penicillin, and 100 mg/ml streptomycin. SILAC-labeling of MIN6 cells and LC-MS/MS-based quantitative proteomics were performed exactly the same way as described previously (23). Antibodies that were used throughout this work were as follow: Ago2 (Cell Signaling C34C6), γ-tubulin (Sigma T6557), Rab3a (Abcam ab3335), Nsf (BD Bioscience), Grp78 (Assay Designs StressGen SPA-826), Slc25a22 (Sigma AV44041), Dicer (Bethyl A301–936A), and Bcl-xL (Cell Signaling #2764). For biochemical fractionation, an eight-step sucrose gradient was performed on MIN6 cells as described previously (23). Briefly, MIN6 cells were washed, pelleted, and resuspended in homogenization buffer containing 5 mm HEPES, 0.5 mm EGTA, and 1× Complete Protease inhibitors (Roche Applied Science) at pH 7.4 and later homogenized. The homogenate was spun at 3000 × g for 10 min at 4 °C, and the post-nuclear supernatant was loaded onto an 8-step discontinuous sucrose density gradient (HEPES-buffered 0.2–2 m sucrose) and centrifuged at 55,000 rpm for 2 h at 4 °C using an MLS50 rotor (Beckman Coulter). Mitochondrial subcellular fractionation was performed as described previously with minor modifications (17). Briefly, 1 × 107 MIN6 cells were washed, resuspended in isotonic mitochondrial buffer (250 mm mannitol, 70 mm sucrose, 1 mm EDTA, 10 mm HEPES pH 7.5), and homogenized. The lysates were spun at 500 × g for 5 min to eliminate unbroken cells, and the supernatant was centrifuged at 10,000 × g for 30 min at 4 °C. The mitochondria-enriched pellet was washed twice and resuspended in mitochondrial buffer for downstream Western blotting analysis.
Transmission Electron Microscopy
Approximately 50 isolated islets were fixed in 2.5% glutaraldehyde in Millonig's buffer (2.26% NaH2PO4 and 2.52% NaOH) for 2 h at 4 °C and then stained in 1.0% osmium tetroxide for 1 h. After dehydration in ethanol, islets were embedded in AGAR 100 (Oxfors Instruments Nordiska AB, Sweden), sectioned (70–90 nm thick), placed on copper grids, and contrasted with uranyl acetate and lead citrate. Imaging was performed on JEM 1230 electron microscope (JEOL-USA, Inc., MA), and micrographs were analyzed with respect to mitochondrial morphology.
Analytic Procedures
Insulin measurements from plasma and sucrose gradient fractions were measured by radioimmunoassay (Millipore), blood glucose was measured as described (18). In vivo glucose or insulin tolerance tests were performed after a 6-h fast and injected intraperitoneally with either glucose (1 g/kg body weight) or insulin (0.75 units/kg body weight), respectively.
Statistical Analysis
All qRT-PCR results are expressed as the mean ± S.E. Comparisons between data sets with two groups were evaluated using an unpaired Student's t test. A p value of ≤0.05 was considered statistically significant. The correlation plots were performed using GraphPad Prism.
Results
miR-184 Is Regulated According to Glucose Metabolism
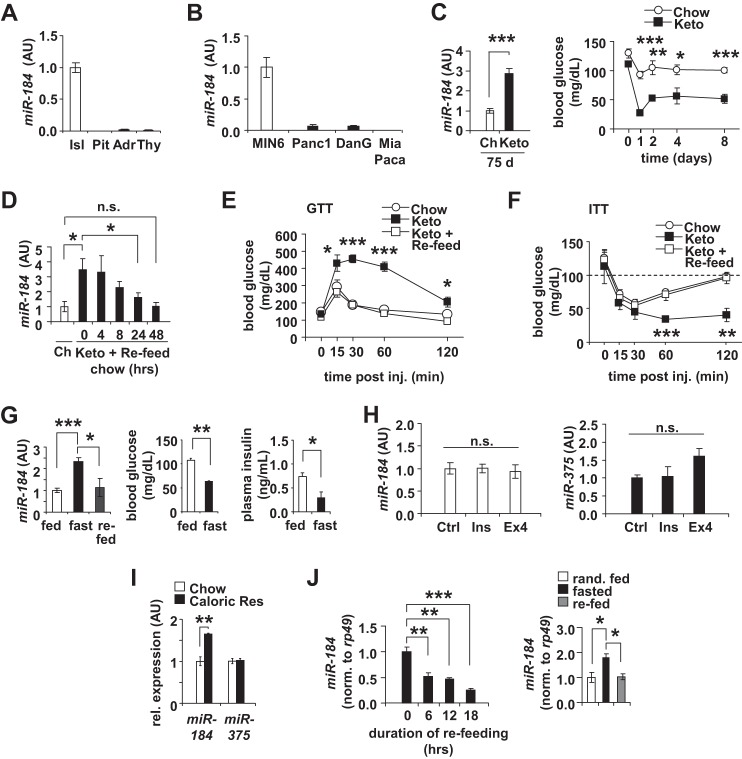
miR-184 is well expressed in the islets when compared with other endocrine tissues such as pituitary, adrenal, and thyroid and more specifically enriched in the MIN6 β-cell line compared with other cell lines of exocrine pancreas (Fig. 1, A and B). Consistent with our previous observation, long term administration of the ketogenic diet for 75 days to C57BL/6 wild-type mice induced ∼3-fold over-expression of miR-184 in their islets (13) (Fig. 1C). In addition, after reverting wild-type mice fed a ketogenic diet for 25 days back to normal chow, miR-184 expression was restored to normal levels in 24 h (Fig. 1D). Moreover, returning mice to a normal chow diet also reversed the effects on both glucose tolerance and insulin sensitivity (Fig. 1, E and F). To further test the effect of nutrient intake on the expression of this miRNA, we next fasted C57BL/6 mice for 30 h, which lowered both glucose and insulin levels. During this fasting phase, we observed a significant increase in miR-184 in isolated islets from these animals, and this increase was normalized 24 h after re-feeding the fasted mice (Fig. 1G). To test whether circulating factors impacting energy homeostasis contribute to changes in miR-184 expression, we treated MIN6 cells with either 100 nm insulin or 20 nm exendin-4 (Ex4) for 48 h and observed no changes in miRNA expression by qRT-PCR (Fig. 1H). In line with fasting, feeding mice with a caloric-restricted diet over a period of 25 days also resulted in an enhanced expression of miR-184, whereas the levels of miR-375 remained unchanged in the isolated islets from these mice (Fig. 1I). Moreover, it has been previously shown that miR-184 was silenced in both the pancreatic islets of type 2 diabetic human subjects compared with non-diabetic controls and in Aplysia sea snails after administration of serotonin in the central nervous system, suggesting its functional role may be conserved a great distance (13, 19). miR-184 is also abundantly expressed in Drosophila, a widely used model for the study of metabolism and longevity; however, its precise functional role in this species remains unknown (20–22). To determine whether miR-184 is also regulated in response to metabolic stimuli in Drosophila, we first provided a sucrose-rich diet to flies after a 16-h fasting period. miR-184 expression significantly decreased 6 h after initiating this diet, indicating that the silencing of this miRNA as glucose metabolism increases is strongly conserved (Fig. 1J). Consistent with our observations in isolated mouse islets, fasting of flies also induced the expression of this miRNA, and upon re-feeding, its levels were normalized (Fig. 1J).
FIGURE 1.
miR-184 is regulated according to glucose metabolism. A, qRT-PCR analysis of relative miR-184 expression in isolated pancreatic islets (Isl), pituitary (Pit), adrenal gland (Adr), and thyroid (Thy) from 10-week-old C57BL/6 mice. AU, arbitrary units. B, qRT-PCR analysis of relative miR-184 expression in several pancreatic cell lines including MIN6, Panc1, DanG, and MiaPaca. C, qRT-PCR analysis of miR-184 in isolated islets of 10-week-old WT mice on either a normal chow (Ch) or ketogenic (Keto) diet for 75 days (n = 4). Random blood glucose levels were decreased after 1 day on ketogenic diet. Results are presented as mean ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001. D, qRT-PCR analysis of miR-184 in isolated islets of 10-week-old WT mice on either normal chow or ketogenic diets for 25 days. Mice on the ketogenic diet were reverted to normal chow for 0–48 h (n = 4). n.s., not significant. E, blood glucose levels during a glucose tolerance test (GTT) from 10-week-old C57BL/6 mice on normal chow (Chow), ketogenic diet (Keto), and normal chow after 25 days on ketogenic diet (Keto + re-feed) (n = 4). F, blood glucose levels during an insulin tolerance test (ITT) from 10-week-old C57BL/6 mice on normal chow (Chow), ketogenic diet (Keto), and normal chow after 25 days on ketogenic diet (Keto + re-feed) (n = 4). G, qRT-PCR analysis of miR-184 and miR-375 in isolated islets of 10 weeks old WT mice fasted for 24 h. Fed and fasted blood glucose and insulin parameters measured before sacrifice (n = 4). H, qRT-PCR analysis of miR-184 and miR-375 after treating MIN6 cells with either 100 nm insulin or 20 nm of exendin-4 (Ex4) for 48 h (n = 3–4). I, qRT-PCR analysis of miR-184 in isolated islets of 10-week-old WT mice on either normal chow or a low calorie diet for 25 days (n = 4). J, qRT-PCR analysis of miR-184 in Drosophila after sucrose administration and after fasting and re-feeding. After a 16 h starvation period, flies were fed with food in which the only caloric supplement is sucrose (5%). Fly heads were collected from flies re-fed for 0, 6, 12, and 18 h of sugar administration.
miR-184 and Ago2 Remain Inversely Correlated According to Glucose Metabolism
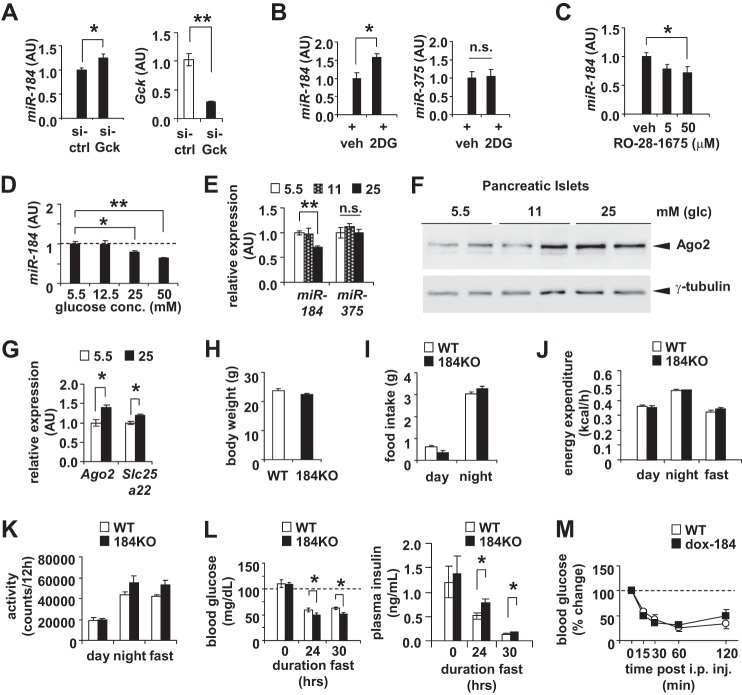
To further understand the role of glucose metabolism in regulating miR-184, we next inhibited glycolysis either by siRNA-mediated knockdown of Gck or treatment of 2-deoxyglucose in MIN6 cells and observed an increase in the expression of miR-184 (Fig. 2, A and B). Conversely, treatment with the glucokinase activator RO-28-1675 resulted in reduced miR-184 levels consistent with increasing glucose metabolism (Fig. 2C). Moreover, the inverse correlation between miR-184 and its targets was also observed upon incubating both MIN6 cells and isolated islets in high and low glucose concentrations (Fig. 2, D–G).
FIGURE 2.
miR-184 and Ago2 remain inversely correlated according to glucose metabolism. A, qRT-PCR analysis of miR-184 in MIN6 cells transfected with either 200 pmol of siRNA targeting glucokinase (si-Gck) or scrambled control (si-Ctrl) for 48 h. AU, arbitrary units. B, qRT-PCR analysis of miR-184 and miR-375 in MIN6 cells after treatment with 2-deoxyglucose (2DG) or vehicle control (veh) for 48 h. C, qRT-PCR analysis of miR-184 in MIN6 cells after receiving 5 or 50 μm glucokinase activator (RO-28–1675) or vehicle control (veh) for 48 h. D, qRT-PCR analysis of miR-184 in MIN6 cells after treatment of 5.5, 12.5, 25, and 50 mm of glucose for 48 h (n = 4 for all concentrations). E, qRT-PCR analysis of miR-184 and miR-375 in isolated islets of 10-week-old WT mice that were treated ex vivo with either 5.5, 11, or 25 mm glucose (n = 4). n.s., not significant. F, Western blotting analysis of Ago2 in the isolated islets of 10-week-old WT mice that were treated ex vivo with 5.5, 11, or 25 mm glucose. G, qRT-PCR analysis of Ago2 and Slc25a22 in isolated islets treated ex vivo with either 5.5 or 25 mm glucose (n = 4). H, body weight measurements of 10-week-old 184KO mice compared with their WT littermate controls (n = 4). I, food intake measurements of 10-week-old 184KO mice compared with their WT littermate controls during day and night (n = 4). J, energy expenditure measurements of 10-week-old 184KO mice compared with their WT littermate controls during the day and night (n = 4). K, activity measurements of 10-week-old 184KO mice compared with their WT littermate controls during the day and night (n = 4). L, fasted blood glucose and plasma insulin levels of 10-week-old 184KO mice compared with their littermate controls (n = 4–6). M, blood glucose levels during an insulin tolerance test (ITT) from 8-week-old WT and dox-184 mice (n = 3–4). Results presented as mean ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Although previous studies have shown that miR-184 mediates the developmental transition of the female germ line in Drosophila as well as neural stem cell differentiation and proliferation in mice, miR-184 knock-out mice (184KO) did not display developmental or behavioral abnormalities as quantified by body weight, food intake, energy expenditure, and locomotor activity in comparison to littermate controls (Fig. 2, H–K) (23, 24). To ultimately test the direct role of miR-184 in maintaining glucose levels, we fasted 184KO mice and observed decreased blood glucose and increased plasma insulin levels (Fig. 2L). These results indicate that miR-184 can directly contribute to systemic glucose levels as glucose metabolism is reduced in the β-cell. Lastly, administration of an insulin tolerance test on miR-184 transgenic mice (dox-184) that overexpress miR-184 in a doxycycline-inducible manner revealed no changes compared with littermate controls indicating increased expression of this miRNA does not significantly contribute to systemic insulin sensitivity (Fig. 2M). Together, our results indicate that it is the regulation of this miRNA in response to changes in energy metabolism that is conserved from mice to flies.
miR-184 Is Regulated upon Inhibition of Glucose Metabolism by Tunicamycin
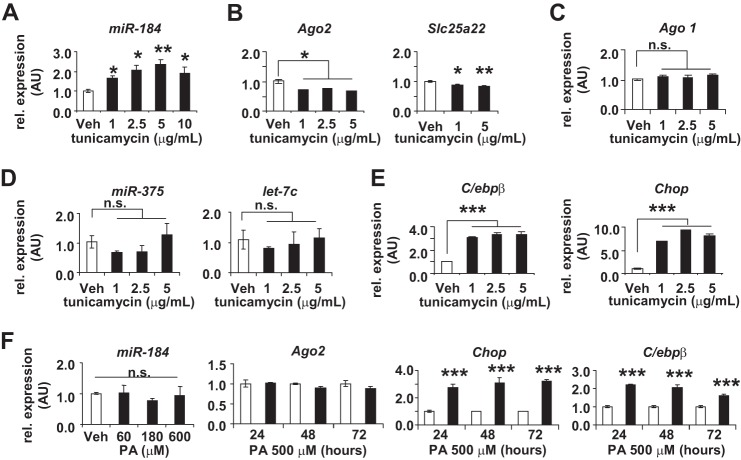
To further test whether inhibition of glucose metabolism by other mechanisms can impact the expression of miR-184 and its targets, we treated MIN6 cells with the N-linked glycosylation blocker, tunicamycin (25). At all concentrations of a dose-response curve, miR-184 expression was significantly increased compared with vehicle alone, as was the expression of the endoplasmic reticulum stress-related genes, C/ebpβ and Chop (Fig. 3, A and E). Again consistent with Ago2 and Slc25a22 being targeted by miR-184, the target gene expression decreased after administration of tunicamycin compared with vehicle alone (Fig. 3B), whereas the expression of Ago1 and other miRNAs including miR-375 and let-7c did not change (Fig. 3, C and D). The robustness of the inverse correlation between miR-184 and its target Ago2 in response to changes in glucose metabolism suggests an important role for these genes in the maintenance of β-cell function. Although treatment of the MIN6 cells with palmitic acid also induced expression of endoplasmic reticulum stress-related genes, neither miR-184 nor Ago2 levels were affected, further supporting the effect of glucose metabolism on their expression (Fig. 3F). Together, these results strongly implicate the regulation of miR-184 and its targets according to glucose metabolism.
FIGURE 3.
miR-184 is regulated upon inhibition of glucose metabolism by tunicamycin. qRT-PCR analysis of miR-184 (A) Ago2 and Slc25a22 (B), Ago1 (C), miR-375 and let-7c (D), and C/ebpβ and Chop (E) mRNA expression in MIN6 cells treated with increasing concentrations of tunicamycin for 48 h (n = 4 for each concentration). AU, arbitrary units; Veh, vehicle. n.s., not significant. F, qRT-PCR analysis of miR-184 in MIN6 cells treated with increasing concentrations of palmitic acid (PA) for 48 h and the levels of Ago2, C/ebpβ, and Chop expression measured after treating MIN6 cells with 500 μm PA over time (n = 4 for each concentration and time point). Results are presented as the mean ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
miR-184 Regulates the β-Cell Secretome
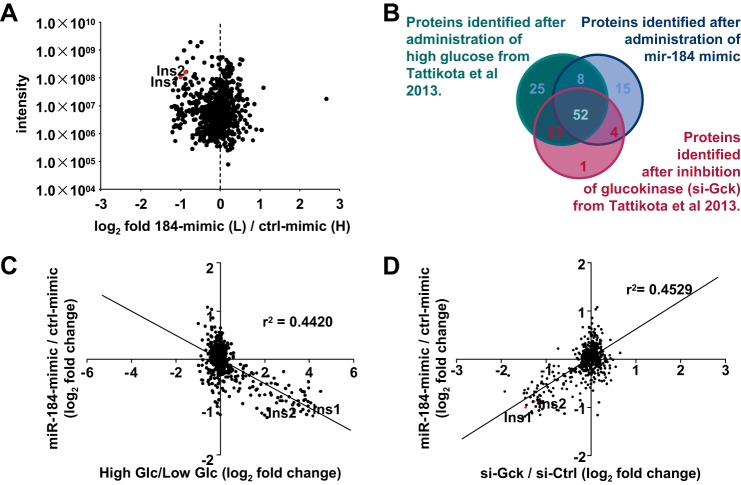
We further addressed the impact of miR-184 on β-cell function by quantifying the secreted proteins after overexpression of this miRNA. SILAC-labeled MIN6 cells that were transfected with miR-184 mimics exhibited a reduced capacity to secrete the previously identified cluster of proteins upon receiving high glucose (Fig. 4A and supplemental Table S1) (26). Importantly, a significant overlap was observed after treatment with miR-184 mimics compared with previous secretome profiles that were identified after high glucose treatment or siRNA-mediated inhibition of glucokinase (Fig. 4, B–D) (26). These observations are consistent with previous results after overexpression of miR-375, indicating several miRNAs may coordinately contribute to the mechanisms leading up to the release of insulin by the β-cell and not to the composition of insulin-containing granules (26). As none of the proteins identified as a part of the secretome is predicted as direct targets of miR-184, these results may suggest abundant miRNAs may impact glucose sensing, mitochondrial metabolism or localization, ion channel function, or the recruitment or fusion of vesicles to the plasma membrane.
FIGURE 4.
miR-184 regulates the β-cell secretome. A, identification of secreted factors that were inhibited after a high glucose stimulus of 184-mimic-transfected “light” compared with Ctrl-mimic transfected “heavy” MIN6 cells after SILAC labeling. B, Venn diagram representation of the secreted factors of MIN6 cells transfected with 184-mimic (from the current study) and from MIN6 cells treated with high/low glucose and si-Gck (taken from Tattikota et al. (26)) having a cutoff value of 0.5 log 2-fold change. C and D, intersection of the secretome profiles of MIN6 cells transfected with 184-mimic (from the current study) and of MIN6 cells treated with high/low glucose and si-Gck (taken from Tattikota et al. (26)) displays an inverse and positive correlation, respectively.
miR-184 Regulates Mitochondrial Respiration
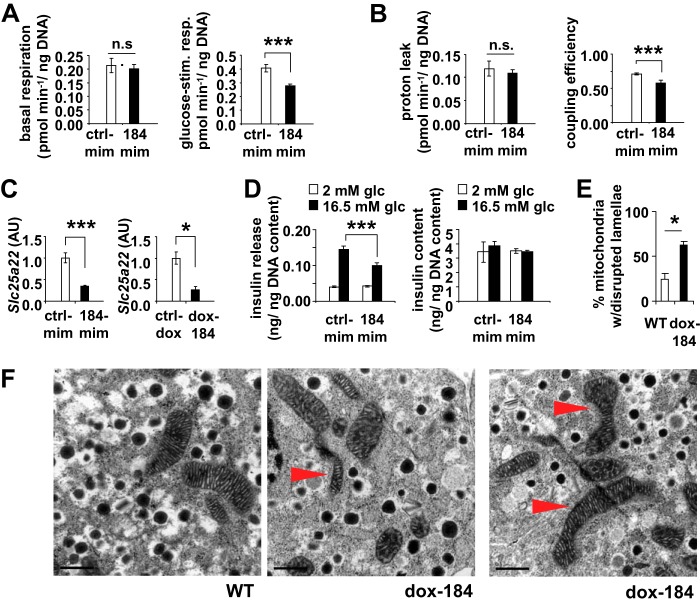
We previously identified the mitochondrial gene Slc25a22 as a target of miR-184 and, therefore, investigated the effect of this miRNA on cellular respiration (13). Transfection of miR-184 mimics in MIN6 cells resulted in a significant decrease of mitochondrial coupling efficiency, and this reduction was caused by reduced glucose-stimulated cellular respiration and not altered mitochondrial proton leak (Fig. 5, A and B). Because the basal respiration remained unchanged, these observations indicate that miR-184 regulates secretion via mitochondrial substrate flux in the β-cell, possibly by suppressing validated targets such as Slc25a22 at the mitochondria (Fig. 5, C and D) (13). Furthermore, transmission electron microscopic analysis of the islets isolated from dox-184 mice revealed a number of morphological alterations including disruption of the lamellae within the mitochondria (Fig. 5, E and F) (13). Previous studies have associated these changes with the loss of cytochrome c-4CYS and mitochondrial function (27). Additionally, a small reduction in the number of mitochondria per cell was observed in dox-184 β-cells (p = 0.10, quantified from transmission electron microscopy images at 8000 magnification: mean (WT) = 22.5 mitochondria/cell; mean (dox-184) = 18.5 mitochondria/cell; 25 cells from each set pooled from n = 3 mice). Together these results suggest that miR-184 may impact the β-cell secretome by targeting Slc25a22 and ultimately mitochondrial respiration.
FIGURE 5.
miR-184 impacts mitochondrial respiration. Measurements of basal respiration and glucose-stimulated respiration (A), proton leak and mitochondrial coupling efficiency (n = 3) in MIN6 cells transfected with either control or 184-mimic (B), and qRT-PCR analysis of miR-184-targeted gene Slc25a22 in MIN6 cells transfected with either control or 184-mimic and in the islets of dox-184 transgenic mice (C), respectively. n.s., not significant; AU, arbitrary units. D, reduced glucose-stimulated insulin secretion in MIN6 cells transfected with either control or 184-mimic. Results are presented as the mean ± S.E. *, p < 0.05; ***, p < 0.001. E, analysis of mitochondria from the beta cells within the islets isolated from WT ctrl-dox and dox-184 mice that received 1 mg/ml doxycycline for 15 days. Data are represented as the percentage of mitochondria with disrupted lamellae. F, transmission electron microscopic (TEM) images of the beta cells within the islets from WT and dox-184 mice that received 1 mg/ml doxycycline for 15 days. Scale bar 0.5 μm.
Ago2 Is Localized at the Mitochondria in MIN6 Cells
As recent studies have established a role for Ago2 within the mitochondria (28), we next examined the localization of Ago2 in MIN6 cells. Ago2 was found abundant in eluted fractions independent of the insulin peak and the granule markers Nsf and Rab3a, suggesting it is not enriched at the secretory granules (Fig. 6A). Western blotting identified Ago2, Slc25a22, and Bcl-xL were present in the mitochondria-containing fractions after subcellular purification (29, 30) (Fig. 6B). siRNA-mediated knockdown of Ago2 in MIN6 cells further confirmed its expression in both the cytoplasmic and mitochondrial fractions (Fig. 6C). These data suggest that miR-184 may impact the growth and function of the β-cell by targeting genes present at the mitochondria including Ago2 and Slc25a22.
FIGURE 6.
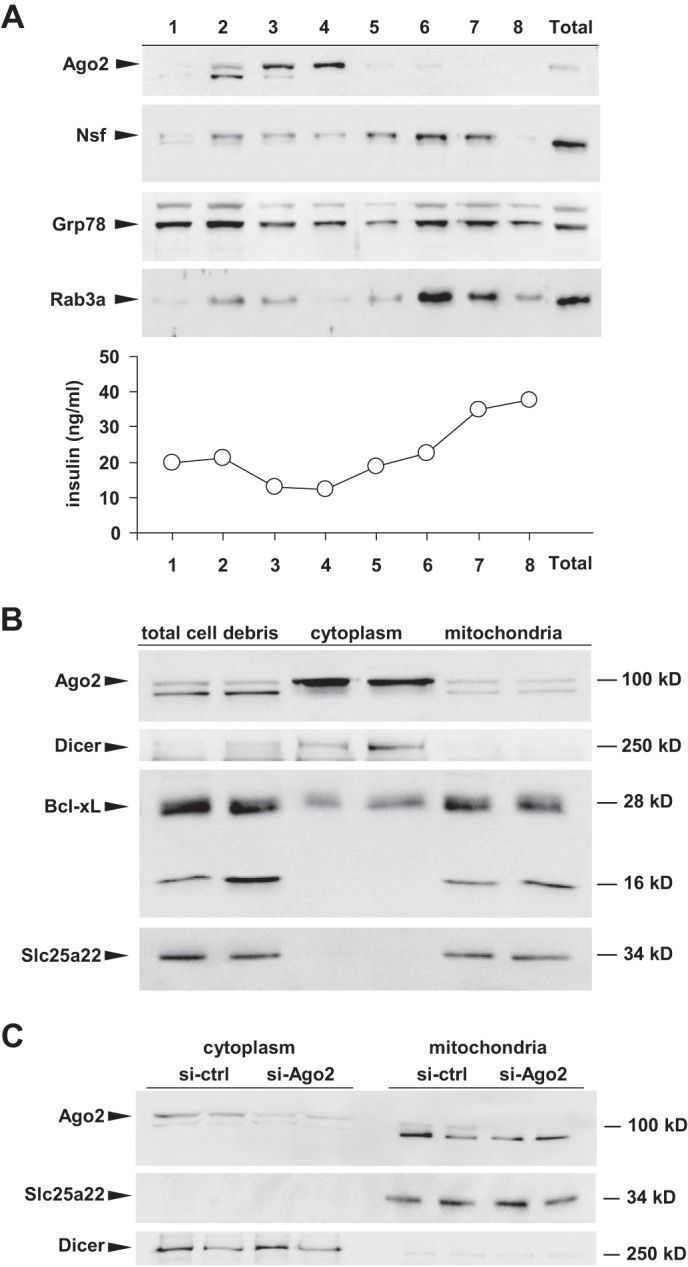
Localization of Ago2 at the mitochondria in MIN6 cells. A, Western blotting analysis and insulin quantification by radioimmunoassay of the fractions derived from a discontinuous sucrose gradient of MIN6 cells. B, Western blotting analysis of cytoplasmic and mitochondrial fractions for Ago2, Bcl-xL, Dicer, and Slc25a22. C, Western blotting analysis of cytoplasmic and purified mitochondria for Ago2, Dicer, and Slc25a22 after si-RNA mediated knockdown of Ago2 in MIN6 cells.
Restoration of Ago2 in the Presence of miR-184 Maintains Normal Glucose Homeostasis
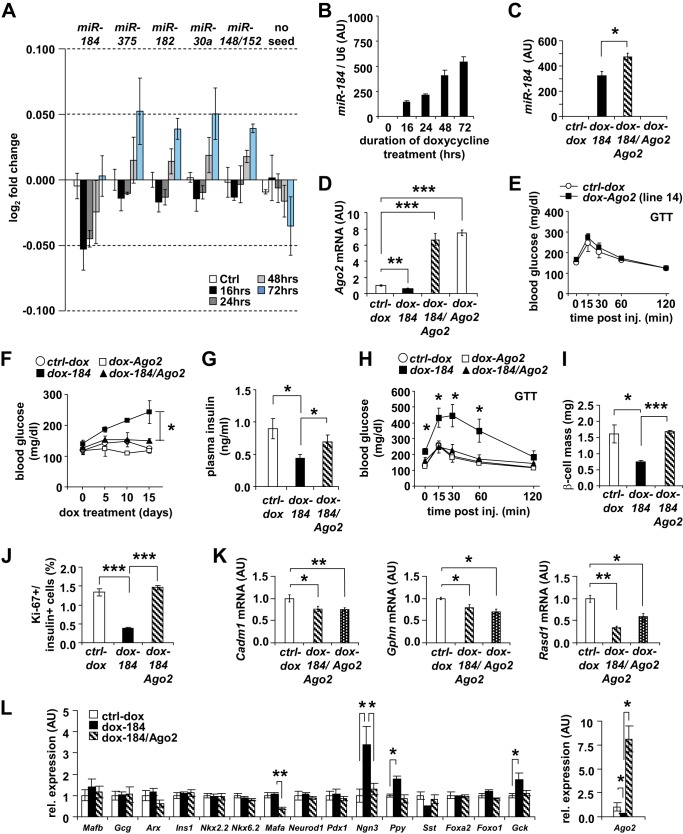
To investigate the impact of altering expression of miR-184 on the β-cell transcriptome, we performed gene expression analysis using Illumina mouse W2vG arrays. The murine MIN6 β-cell line was transfected with two plasmids expressing 1) the reverse tetracycline transactivator (rtTA) and 2) a genomic fragment encompassing the miR-184 precursor under the control of the tet operon (184-tetO). Upon treating the cells with 1 μg/ml doxycycline (dox), we observed a progressive increase of miR-184 after 16 h of dox administration, whereas genes containing miR-184-seed matches significantly decreased their expression compared with genes without seed matches (Fig. 7, A and B). The down-regulation of miR-184 targets was transient over time, in line with miR-184 targeting Ago2. In addition, genes containing matches for the seeds of three additional miRNAs that are abundant in this cell line including miR-375, 182, 30a, and 148a/152, were progressively up-regulated compared with genes without seed matches for these miRNAs, consistent with the idea that miR-184 can impact miRNA-mediated regulation via the down-regulation of Ago2 (Fig. 7A).
FIGURE 7.
Restoration of Ago2 in the presence of miR-184 maintains normal glucose homeostasis. A, Illumina-based gene expression array analysis of the targets of miRs-184, -375, -182, and -30a in MIN6 cells overexpressing miR-184 after 16, 24, 48, and 72 h of doxycycline induction compared with controls (n = 406, 1087, 1575, 1727, and 6679 for miR-184, -375, -182, -30a, 148a/152, respectively, and no seed). AU, arbitrary units. B, miR-184 overexpression in MIN6 cells induced by 1 μg/ml doxycycline between 0 and 72 h. C, qRT-PCR analysis of miR-184 in isolated islets of 12-week-old dox-184, dox-184/Ago2, and dox-Ago2 transgenic mice compared with their WT ctrl-dox littermates treated with 1 mg/ml doxycycline in their drinking water for 15 days (n = 4–6). D, qRT-PCR analysis of Ago2 mRNA in isolated islets of 12-week-old dox-184, dox-184/Ago2, and dox-Ago2 transgenic mice compared with their WT ctrl-dox littermates treated with 1 mg/ml doxycycline in their drinking water for 15 days (n = 4–6). E, blood glucose levels during a glucose tolerance test (GTT) on 12-week-old dox-Ago2 and WT ctrl-dox littermates that were not treated with doxycycline (n = 4). F, random blood glucose levels of 12-week-old mice compared with dox-184, dox-184/Ago2, and dox-Ago2 transgenic mice compared with their WT ctrl-dox littermates treated with 1 mg/ml doxycycline in their drinking water for 15 days (n = 12). G, random insulin levels of 12-week-old dox-184 and dox-184/Ago2 and WT ctrl-dox littermates treated with 1 mg/ml doxycycline in their drinking water for 15 days (n = 12–14). H, blood glucose levels during a glucose tolerance test on 12-week-old dox-184, dox-184/Ago2, and dox-Ago2 transgenic mice compared with their WT ctrl-dox littermates treated with 1 mg/ml doxycycline in their drinking water for 15 days (n = 4–6). I, quantification of β-cell mass in 12-week-old dox-184, dox-184/Ago2 mice compared with WT ctrl-dox littermates treated with 1 mg/ml dox in their drinking water for 15 days (n = 3). J, quantification of Ki-67+ β-cells in 12-week-old dox-184, dox-184/Ago2 mice compared with WT ctrl-dox littermates (n = 3). K, qRT-PCR analysis of miR-375 targeted genes Cadm1, Gphn, and Rasd1 in isolated islets of 12-week old dox-184, dox-184/Ago2, and dox-Ago2 transgenic mice compared with their WT ctrl-dox littermates treated with 1 mg/ml doxycycline in their drinking water for 15 days (n = 6). L, qRT-PCR analysis of islet-specific marker genes in isolated islets of 12-week-old dox-184, dox-184/Ago2 transgenic mice compared with their WT ctrl-dox littermates treated with 1 mg/ml doxycycline in their drinking water for 15 days (n = 6). Results are presented as mean ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To further test the physiologic relevance of the targeting of Ago2 by miR-184, we next crossed two previously generated transgenic mouse models that allowed overexpression of Ago2 and miR-184 in the pancreatic β-cell in a doxycycline-inducible manner (dox-Ago2 and dox-184, respectively) (13). Doxycycline-dependent Ago2 expression was possible in the presence of miR-184 in dox-184/Ago2 animals as the Ago2 transgene lacked the 3′-UTR harboring the miR-184-seed sequence (13). Dox-184/Ago2 mice exhibited comparable expression of both miR-184 and Ago2 to their littermate controls, dox-184 and dox-Ago2, respectively (Fig. 7, C and D). Although dox-Ago2 mice displayed normal random glucose and glucose tolerance after treatment of doxycycline, dox-184 animals were hyperglycemic as a result of decreased circulating insulin as previously shown (Fig. 7, E–G) (13). Interestingly, dox-184/Ago2 mice maintained normal steady-state glucose and insulin levels and glucose tolerance in contrast to dox-184 mice, indicating that the maintenance of Ago2 expression in the presence of miR-184 restored normal glucose control (Fig. 7, F–H). Furthermore, pancreatic β-cell mass and proliferation rate were also restored in dox-184/Ago2 mice consistent with the maintenance of glucose homeostasis in these animals (Fig. 7, I and J) (13). To determine whether the capacity to maintain miRNA-mediated gene regulation by Ago2 remained intact in dox-184/Ago2 mice, we next assessed the expression of miR-375-targeted genes in isolated islets. Expression of Cadm1, Gphn, and Rasd1 was lower in islets isolated from dox-184/Ago2 mice and islets of dox-Ago2 animals compared with littermate controls (Fig. 7K). Although the mechanism of this rescue is not precisely clear, these results show Ago2-mediated gene regulation is maintained in these animals and may indicate the function of other abundant miRNAs including miR-375 countering the action of miR-184. To determine whether overexpression of miR-184 leads to de-differentiation of the β-cell, a broad range of islet marker genes was quantified by qRT-PCR (Fig. 7L). Although marked increases in Ngn3, Ppy, and Gck were observed in islets from dox-184 mice, suggesting a degree of de-differentiation, the majority of genes remained unchanged, indicating miR-184 is not a significant contributor to β-cell differentiation (Fig. 7L).
Discussion
Animals adapt their physiology to changes in nutrient intake through specific molecular mechanisms. Interestingly, organisms at all levels of complexity display increased longevity in response to caloric restriction, suggesting that the fundamental components of these pathways is highly conserved (31, 32). During fasting conditions, it has long been known that the pancreatic β-cell reduces its output of secreted insulin, indicating a central role for glucose metabolism in β-cell physiology (1). We recently showed that miR-184, a highly conserved and abundant miRNA expressed in the β-cell, regulates compensatory proliferation and secretion during insulin resistance (13). In contrast to all other miRNAs that are expressed in this cell type, miR-184 was silenced with the onset insulin resistance and then re-emerged upon administration of a low carbohydrate, ketogenic diet. These observations, after administration of either high fat or ketogenic diets, implicate changes in nutrient intake and insulin sensitivity in the regulation of this miRNA.
Here in this study we follow up on these observations to address the specific impact of nutrient metabolism on the miRNA pathway and show that expression of both miR-184 and its target Ago2 are sensitive to changes in glucose metabolism in the β-cell. Although miR-184 levels in mouse islets increased after a ketogenic diet, the levels of this miRNA were restored within 24 h after reversion to a normal chow diet. In contrast, placing mice on a high fat diet required several weeks for inducing the silencing of this miRNA (13). These results suggest that the β-cell may respond more acutely to specific stimuli such as glucose than to changes in insulin sensitivity. Although the knockouts of miR-184 and Ago2 in the β-cell both exhibit no changes during an insulin tolerance test, these results indicate the miRNA pathway in this cell type may not significantly contribute to systemic insulin sensitivity (13, 18). The functional link between miR-184 and glucose metabolism in the β-cell is further supported by the relatively specific expression profile of this miRNA. In contrast to other abundant miRNAs in the β-cell such as miR-375, miR-184 does not appear to be expressed in the pituitary, adrenal gland, or exocrine pancreas. This limited expression profile may highlight the importance of this miRNA in the regulation of β-cell function according to changes in energy homeostasis.
Importantly, it has long been known that haploinsufficiency of Gck resulted in decreased insulin release, underlining the essential role of glucose sensing and metabolism in mediating secretion by the β-cell (33). Our observation that the knockdown of glucokinase resulted in increased expression of miR-184 indicates that the β-cells activate the expression of this miRNA to suppress insulin release when either glucose sensing or glycolysis is attenuated. Both 2-deoxyglucose and tunicamycin treatments had similar effects, further underlining the role of glucose metabolism in the regulation of miR-184. Importantly, although both of these reagents are also widely known to induce endoplasmic reticulum stress and the unfolded protein response, the absence of any effect of palmitic acid suggests that the endoplasmic reticulum stress pathway does not significantly contribute to changes in miR-184 expression (34).
The precise role of the miRNA pathway remains to be established; however, a recurring theme in many published studies is its function in adaptive stress responses (14). In light of the robust inverse correlation between miR-184 and Argonaute2 expression, our observations from the dox-184/Ago2 mice would also suggest that many aspects of the involvement of these two genes in glucose homeostasis remain to be described. It is unclear whether the normoglycemia and glucose tolerance observed in these mice results from Ago2-mediated gene silencing or a non-canonical effect of Ago2 on gene expression via its localization in the nucleus, mitochondria, or stress granule of the β-cell (28, 35, 36). Nonetheless, the restoration of normal glucose homeostasis and β-cell mass in the dox-184/Ago2 model provides further support for Ago2 as a biologically relevant target of miR-184 using an in vivo system.
Although our results continue to underline miR-184 as a potent inhibitor of insulin release, the precise actions of its identified targets remain unclear (13, 26). Inhibition of the two validated targets of this miRNA, Ago2 and Slc25a22, result in contradictory effects on glucose-stimulated insulin secretion and may suggest a hierarchical presence among targets. Although miR-184 expression is elevated as glucose metabolism is attenuated in the β-cell, it is possible the suppression of Slc25a22 under these conditions may have a more appreciable effect than the suppression of Ago2 in the absence of any glucose stimulus. Conversely, we observed the silencing of miR-184 in the presence of high extracellular glucose concentrations. Although increased expression of both Ago2 and miR-375 will contribute to suppress secretion, these genes mediate compensatory proliferation as metabolic demand increases and may highlight another functional hierarchy within this cell type (13, 18). In light of the proliferative effect of glucose on the β-cell, our results continue to indicate the miRNA pathway facilitates growth and in turn compromises insulin release. It is possible that as the need for insulin rises, proliferation is prioritized and the suppression of secretion is ultimately inconsequential as more cells are present to alleviate metabolic demand. Importantly, it remains to be precisely described how the β-cell mediates the energy balance between cell size and growth, granule synthesis, and insulin release.
Interestingly, recent studies have shown Ago2 may complex miRNAs independent of target mRNAs according to metabolic state (38). Future studies may identify the majority if not the full extent of β-cell transcripts critical to the regulation of its growth and function complexed to miRNAs and RNA-binding proteins such as Ago2 largely during periods of increased metabolic demand such as the post-prandial state. The occupancy of these β-cell genes by RNA-protein complexes may in turn be determined by metabolic cues such as shifts in systemic glucose levels (39). The presence of miRNAs and RNA-binding proteins on these β-cell genes may act to “stall” their expression until the demand ceases, at which time miRNAs and associated binding proteins are “de-recruited” to an inactive and uncomplexed state. The miRNA pathway may ultimately act as an energy-efficient means of modulating gene expression according to changes in metabolic demand rather than solely implementing de novo transcription to promote expression or degradation of critical mRNAs to silence genes.
As miR-184 is highly conserved, future studies may also address how this miRNA impacts energy homeostasis in other model organisms including Aplysia and Drosophila to decipher the most fundamental aspects glucose metabolism (22). Although progress is currently hindered due in part to the absence of accurate annotations of orthologous genes between Drosophila and mammalian species, several key components of the insulin/Igf-like signaling (IIS) pathway are conserved. In addition, the transcription factor dFOXO has been implicated in metabolic processes in many organisms; however, the extent to which its function is conserved between species remains unclear (37). Likewise, Ago1 is the established mediator of miRNA-mediated gene regulation in Drosophila species, further suggesting that although the functional role of miR-184 may be conserved between species, the transcription factors promoting its expression as well as its direct targets may not be.
Future studies emphasizing the identification of the key factors regulating energy homeostasis, which are conserved between species, will have strong implications on the study of longevity and metabolic disease. Our results shown using Drosophila may play a key role in elucidating the fundamental relationship between miR-184 and energy homeostasis and how these genes can impact the aging process or the onset of insulin resistance and diabetes.
Author Contributions
S. G. T. and M. N. P. conceived this study. S. G. T., T. R., J. H., A. K., U. D. K., V. K. P., M. S., H.-H. W., I. G. M., L. E., M. S., R. P. Z., M. Z., S. K., M. T., M. J., M. R. F., and M. N. P. designed and performed the experiments with help from all authors. S. G. T. and M. N. P. wrote the manuscript.
Supplementary Material
Acknowledgments
We thank P. Aranda Chale, M. Schwarz, S. Jia, O. Gustafsson, B-M Nilsson, R. Schweiker, J. Rossius, D. Aberdam, R. Shalom-Feuerstein, T. Willnow, M. Gotthardt, A. Sporbert, M. Richter, and the MDC Microscopy Core facility, B. Jerchow, and the MDC Animal Core facility for assistance in the conduct of this work.
This work was supported by the Helmholtz Gemeinschaft, an ERC starting grant (IsletVasc 260744), the Fritz Thyssen Stiftung, the Deutsches Zentrum fuer Herz-Kreislauf Forschung, E.V. (DZHK), Israeli Science Foundation (ISF#840/2014 to S. K.), and the Swedish Research Council and Swedish Diabetes Association (to L. E.). The authors do not declare any competing financial interests.

This article contains supplemental Table S1.
The NCBI Gene Expression Omnibus (GEO) and Sequence Read Archive (SRA) accession numbers for the referenced array and sequencing data are GSE46623.
- miRNA
- microRNA
- Gck
- glucokinase
- 184KO
- miR-184 knock-out
- dox
- doxycycline
- qRT
- quantitative real-time
- SILAC
- stable isotope labeling by amino acids in cell culture.
References
- 1. Grey N. J., Goldring S., Kipnis D. M. (1970) The effect of fasting, diet, and actinomycin D on insulin secretion in the rat. J. Clin. Invest. 49, 881–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ashcroft S. J., Randle P. J. (1968) Control of insulin release by glucose. Proc. R. Soc. Med. 61, 814–815 [PMC free article] [PubMed] [Google Scholar]
- 3. Alonso L. C., Yokoe T., Zhang P., Scott D. K., Kim S. K., O'Donnell C. P., Garcia-Ocaña A. (2007) Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes 56, 1792–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonner-Weir S., Deery D., Leahy J. L., Weir G. C. (1989) Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes 38, 49–53 [DOI] [PubMed] [Google Scholar]
- 5. Chick W. L. (1973) Beta cell replication in rat pancreatic monolayer cultures. Effects of glucose, tolbutamide, glucocorticoid, growth hormone, and glucagon. Diabetes 22, 687–693 [DOI] [PubMed] [Google Scholar]
- 6. Porat S., Weinberg-Corem N., Tornovsky-Babaey S., Schyr-Ben-Haroush R., Hija A., Stolovich-Rain M., Dadon D., Granot Z., Ben-Hur V., White P., Girard C. A., Karni R., Kaestner K. H., Ashcroft F. M., Magnuson M. A., Saada A., Grimsby J., Glaser B., Dor Y. (2011) Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab. 13, 440–449 [DOI] [PubMed] [Google Scholar]
- 7. Danial N. N., Walensky L. D., Zhang C.-Y., Choi C. S., Fisher J. K., Molina A. J., Datta S. R., Pitter K. L., Bird G. H., Wikstrom J. D., Deeney J. T., Robertson K., Morash J., Kulkarni A., Neschen S., Kim S., Greenberg M. E., Corkey B. E., Shirihai O. S., Shulman G. I., Lowell B. B., Korsmeyer S. J. (2008) Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat. Med. 14, 144–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hija A., Salpeter S., Klochendler A., Grimsby J., Brandeis M., Glaser B., Dor Y. (2014) G0-G1 transition and the restriction point in pancreatic β-cells in vivo. Diabetes 63, 578–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen H., Gu X., Su I.-H., Bottino R., Contreras J. L., Tarakhovsky A., Kim S. K. (2009) Polycomb protein Ezh2 regulates pancreatic β-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev. 23, 975–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krishnamurthy J., Ramsey M. R., Ligon K. L., Torrice C., Koh A., Bonner-Weir S., Sharpless N. E. (2006) p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 443, 453–457 [DOI] [PubMed] [Google Scholar]
- 11. Chen H., Gu X., Liu Y., Wang J., Wirt S. E., Bottino R., Schorle H., Sage J., Kim S. K. (2011) PDGF signalling controls age-dependent proliferation in pancreatic β-cells. Nature 478, 349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meier J. J., Butler A. E., Saisho Y., Monchamp T., Galasso R., Bhushan A., Rizza R. A., Butler P. C. (2008) Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 57, 1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tattikota S. G., Rathjen T., McAnulty S. J., Wessels H.-H., Akerman I., van de Bunt M., Hausser J., Esguerra J. L., Musahl A., Pandey A. K., You X., Chen W., Herrera P. L., Johnson P. R., O'Carroll D., Eliasson L., Zavolan M., Gloyn A. L., Ferrer J., Shalom-Feuerstein R., Aberdam D., Poy M. N. (2014) Argonaute2 mediates compensatory expansion of the pancreatic β cell. Cell Metab. 19, 122–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leung A. K., Sharp P. A. (2010) MicroRNA functions in stress responses. Mol. Cell. 40, 205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mendell J. T., Olson E. N. (2012) MicroRNAs in stress signaling and human disease. Cell 148, 1172–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bartel D. P. (2009) MicroRNAs: target recognition and regulatory functions. Cell. 136, 215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jan Y., Matter M., Pai J. T., Chen Y.-L., Pilch J., Komatsu M., Ong E., Fukuda M., Ruoslahti E. (2004) A mitochondrial protein, Bit1, mediates apoptosis regulated by integrins and Groucho/TLE corepressors. Cell 116, 751–762 [DOI] [PubMed] [Google Scholar]
- 18. Poy M. N., Hausser J., Trajkovski M., Braun M., Collins S., Rorsman P., Zavolan M., Stoffel M. (2009) miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc. Natl. Acad. Sci. U.S.A. 106, 5813–5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rajasethupathy P., Fiumara F., Sheridan R., Betel D., Puthanveettil S. V., Russo J. J., Sander C., Tuschl T., Kandel E. (2009) Characterization of small RNAs in aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron 63, 803–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong X., Hammell M., Ambros V., Cohen S. M. (2009) Immunopurification of Ago1 miRNPs selects for a distinct class of microRNA targets. Proc. Natl. Acad. Sci. U.S.A. 106, 15085–15090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grün D., Wang Y.-L., Langenberger D., Gunsalus K. C., Rajewsky N. (2005) microRNA target predictions across seven Drosophila species and comparison to mammalian targets. PLoS Comput. Biol. 1, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Padmanabha D., Baker K. D. (2014) Drosophila gains traction as a repurposed tool to investigate metabolism. Trends Endocrinol. Metab. 25, 518–527 [DOI] [PubMed] [Google Scholar]
- 23. Iovino N., Pane A., Gaul U. (2009) miR-184 has multiple roles in Drosophila female germ line development. Dev. Cell. 17, 123–133 [DOI] [PubMed] [Google Scholar]
- 24. Liu C., Teng Z.-Q., Santistevan N. J., Szulwach K. E., Guo W., Jin P., Zhao X. (2010) Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 6, 433–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olden K., Pratt R. M., Yamada K. M. (1978) Role of carbohydrates in protein secretion and turnover: effects of tunicamycin on the major cell surface glycoprotein of chick embryo fibroblasts. Cell 13, 461–473 [DOI] [PubMed] [Google Scholar]
- 26. Tattikota S. G., Sury M. D., Rathjen T., Wessels H.-H., Pandey A. K., You X., Becker C., Chen W., Selbach M., Poy M. N. (2013) Argonaute2 regulates the pancreatic β-cell secretome. Mol. Cell. Proteomics 12, 1214–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun M. G., Williams J., Munoz-Pinedo C., Perkins G. A., Brown J. M., Ellisman M. H., Green D. R., Frey T. G. (2007) Correlated three-dimensional light and electron microscopy reveals transformation of mitochondria during apoptosis. Nat Cell Biol. 9, 1057–1065 [DOI] [PubMed] [Google Scholar]
- 28. Zhang X., Zuo X., Yang B., Li Z., Xue Y., Zhou Y., Huang J., Zhao X., Zhou J., Yan Y., Zhang H., Guo P., Sun H., Guo L., Zhang Y., Fu X.-D. (2014) MicroRNA directly enhances mitochondrial translation during muscle differentiation. Cell. 158, 607–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luciani D. S., White S. A., Widenmaier S. B., Saran V. V., Taghizadeh F., Hu X., Allard M. F., Johnson J. D. (2013) Bcl-2 and Bcl-xL suppress glucose signaling in pancreatic β-cells. Diabetes 62, 170–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Casimir M., Lasorsa F. M., Rubi B., Caille D., Palmieri F., Meda P., Maechler P. (2009) Mitochondrial glutamate carrier GC1 as a newly identified player in the control of glucose-stimulated insulin secretion. J. Biol. Chem. 284, 25004–25014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fontana L., Partridge L., Longo V. D. (2010) Extending healthy life span: from yeast to humans. Science 328, 321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Longo V. D., Mattson M. P. (2014) Fasting: Molecular Mechanisms and clinical applications. Cell Metab. 19, 181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Terauchi Y., Takamoto I., Kubota N., Matsui J., Suzuki R., Komeda K., Hara A., Toyoda Y., Miwa I., Aizawa S., Tsutsumi S., Tsubamoto Y., Hashimoto S., Eto K., Nakamura A., Noda M., Tobe K., Aburatani H., Nagai R., Kadowaki T. (2007) Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. J. Clin. Invest. 117, 246–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hotamisligil G. S. (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cernilogar F. M., Onorati M. C., Kothe G. O., Burroughs A. M., Parsi K. M., Breiling A., Lo Sardo F., Saxena A., Miyoshi K., Siomi H., Siomi M. C., Carninci P., Gilmour D. S., Corona D. F., Orlando V. (2011) Chromatin-associated RNA interference components contribute to transcriptional regulation in Drosophila. Nature 480, 391–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leung A. K., Calabrese J. M., Sharp P. A. (2006) Quantitative analysis of Argonaute protein reveals microRNA-dependent localization to stress granules. Proc. Natl. Acad. Sci. U.S.A. 103, 18125–18130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Giannakou M. E., Goss M., Partridge L. (2008) Role of dFOXO in lifespan extension by dietary restriction in Drosophila melanogaster: not required, but its activity modulates the response. Aging Cell. 7, 187–198 [DOI] [PubMed] [Google Scholar]
- 38. La Rocca G., Olejniczak S. H., González A. J., Briskin D., Vidigal J. A., Spraggon L., DeMatteo R. G., Radler M. R., Lindsten T., Ventura A., Tuschl T., Leslie C. S., Thompson C. B. (2015) In vivo, Argonaute-bound microRNAs exist predominantly in a reservoir of low molecular weight complexes not associated with mRNA. Proc. Natl. Acad. Sci. U.S.A. 112, 767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Olejniczak S. H., La Rocca G., Gruber J. J., Thompson C. B. (2013) Long-lived microRNA-Argonaute complexes in quiescent cells can be activated to regulate mitogenic responses. Proc. Natl. Acad. Sci. U.S.A. 110, 157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.