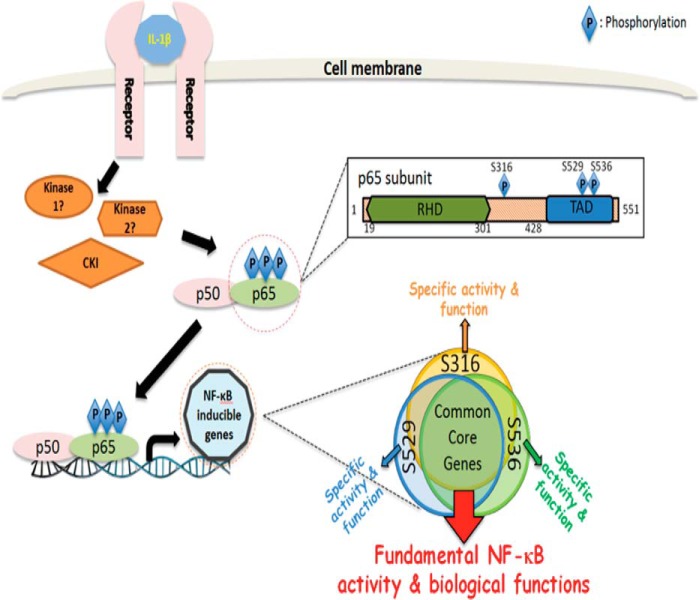
FIGURE 8.
Hypothetical model. We hypothesize that upon treatment with IL-1β, Ser-316, Ser-529, or Ser-536 of p65 are phosphorylated by the same or different kinases, including CKI (inset: magnified image of the precise phosphorylation sites and their location in the p65 structure). Each phosphorylated site regulates the common core (∼80% of genes in our case) of NF-κB dependent genes. Additionally, each site further regulates ∼20% unique subgroups of genes (inset: illustrating common core genes and different specific subgroups of genes that are activated by each of the three phosphorylated sites individually or cooperatively). This regulation pattern not only will allow NF-κB to exhibit its major fundamental biological functions but also further provide NF-κB with more specificity and flexibility, thus leading to diversified NF-κB-driven biological consequences in specific cell systems under specific microenvironments and in response to a variety of different specific stimuli. TAD, transactivation domain.