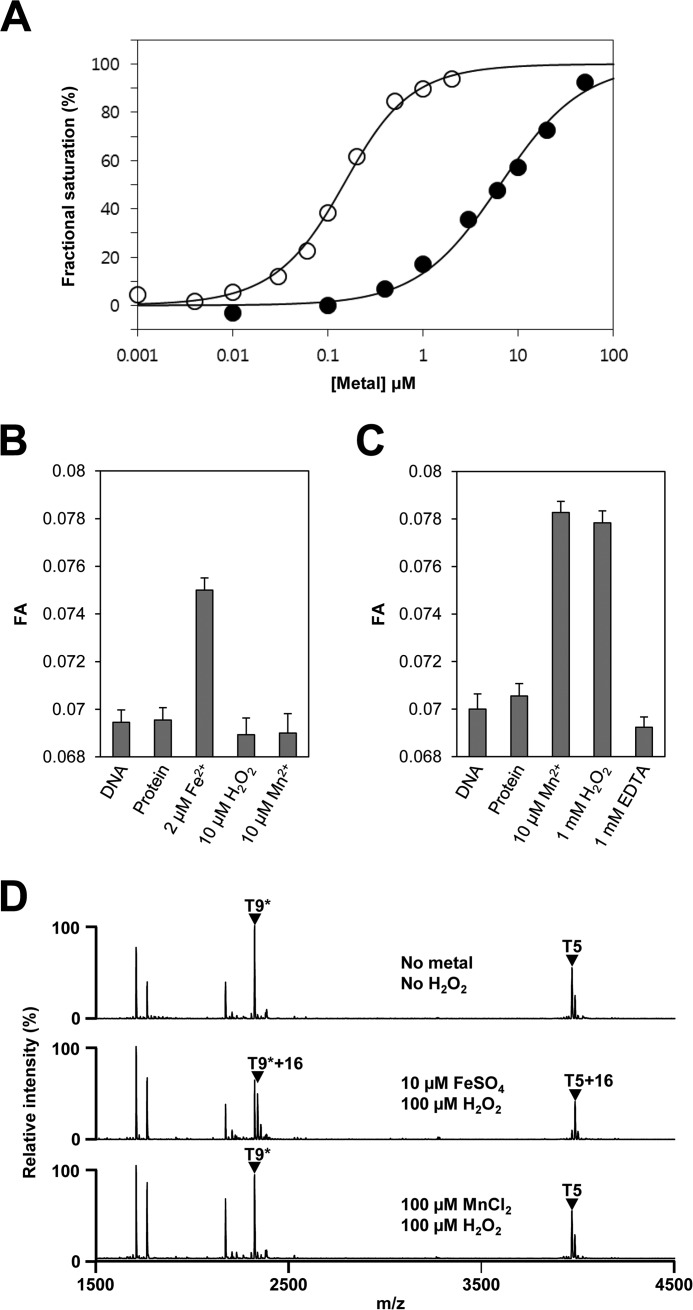
FIGURE 3.
Metal-dependent DNA binding and H2O2-mediated oxidation of PerRSAin vitro. A, metal-dependent DNA binding activity of PerRSA. Various concentrations of metal ions (open circle for Fe2+ and filled circle for Mn2+) are added to samples containing 100 nm DNA and 100 nm active PerRSA dimer, and metal-dependent DNA-binding of PerRSA was monitored by fluorescence anisotropy change. B, sensitivity of PerRSA to H2O2 in the presence of Fe2+. Protein (100 nm active PerRSA:Zn dimer), FeSO4, H2O2, and MnCl2 were sequentially added to buffer A containing 100 nm DNA with an interval of 2 min between each addition, and FA was measured after each addition. 10 μm H2O2 rapidly (<20 s) inactivated PerRSA in the presence of 2 μm Fe2+ and the loss of PerRSA activity was not recovered by the addition of Mn2+. C, sensitivity of PerRSA to H2O2 in the presence of Mn2+. Protein (100 nm active PerRSA:Zn dimer), MnCl2, H2O2, and EDTA were sequentially added to buffer A containing 100 nm DNA with an interval of 2 min between each addition except for 10 min between H2O2 and EDTA, and FA was measured after each addition. PerRSA was insensitive to 1 mm H2O2 for 10 min but lost DNA-binding activity rapidly by addition of 1 mm EDTA. D, metal-dependent oxidation of PerRSA. Oxidation of PerRSA by H2O2 in the presence of 10 μm Fe2+ or 100 μm Mn2+ was monitored by MALDI-TOF MS after tryptic digestion. Note that no cysteine oxidation was observed as judged by fully alkylated T9 peptide (T9*) containing Cys-102 and Cys-105.