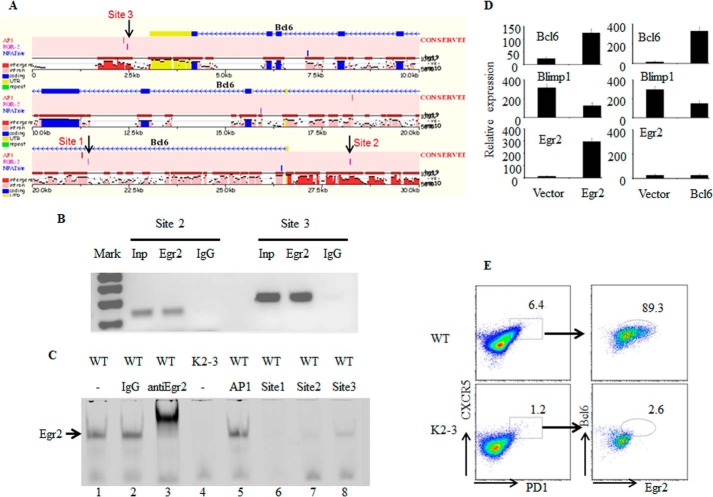
FIGURE 6.
EGR2 directly regulates Bcl6 expression in CD4 T cells. A, identification of three potential binding sites for EGR2 in conserved regions of the Bcl6 locus using Mulan and multiTF (24). B, to determine whether EGR2 interacts with these sites, CD4 T cells from wild type mice were stimulated with anti-CD3 and anti-CD28 for 16 h and used in a ChIP assay with primers flanking two of the potential EGR binding sites (sites 2 and 3) in the Bcl6 locus. Total input DNA and anti-Ig precipitates served as positive and negative controls, respectively. C, interaction of EGR2 with probes derived from the potential binding sites (sites 1, 2, and 3) in the Bcl6 locus by EMSA. The EGR2 consensus binding sequence probe interacted with EGR2 from nuclear extracts of wild type CD4 T cells that were stimulated by anti-CD3 and anti-CD28 antibodies for 16 h (lane 1). The EGR2 probe band can be supershifted by the addition of anti-EGR2 antibody (lane 3) but not control IgG (lane 2). Probes derived from the potential EGR2 binding sites in the Bcl6 locus (sites 1, 2, and 3) (lanes 6–8), but not an AP-1 probe (lane 5), competed for EGR2 binding. Nuclear extract from EGR2/3-deficient CD4 T cells stimulated by anti-CD3 and anti-CD28 for 16 h served as a negative control (lane 4). D, RT-PCR analysis of Bcl6, BLIMP1, and Egr2 in EGR2/3-deficient CD4 T cells 24 h after transduction with control lentivirus or lentivirus encoding EGR2 (left) or BCL6 (right). Error bars, SD. E, BCL6 and EGR2 expression in Tfh cells in WT and CD2-Egr2−/−Egr3−/− (K2-3) mice 14 days after vaccinia virus infection. Data are representative of three (B, C, and E) or two (D) independent experiments. The RT-PCR expression results are presented relative to the expression of β-actin mRNA.