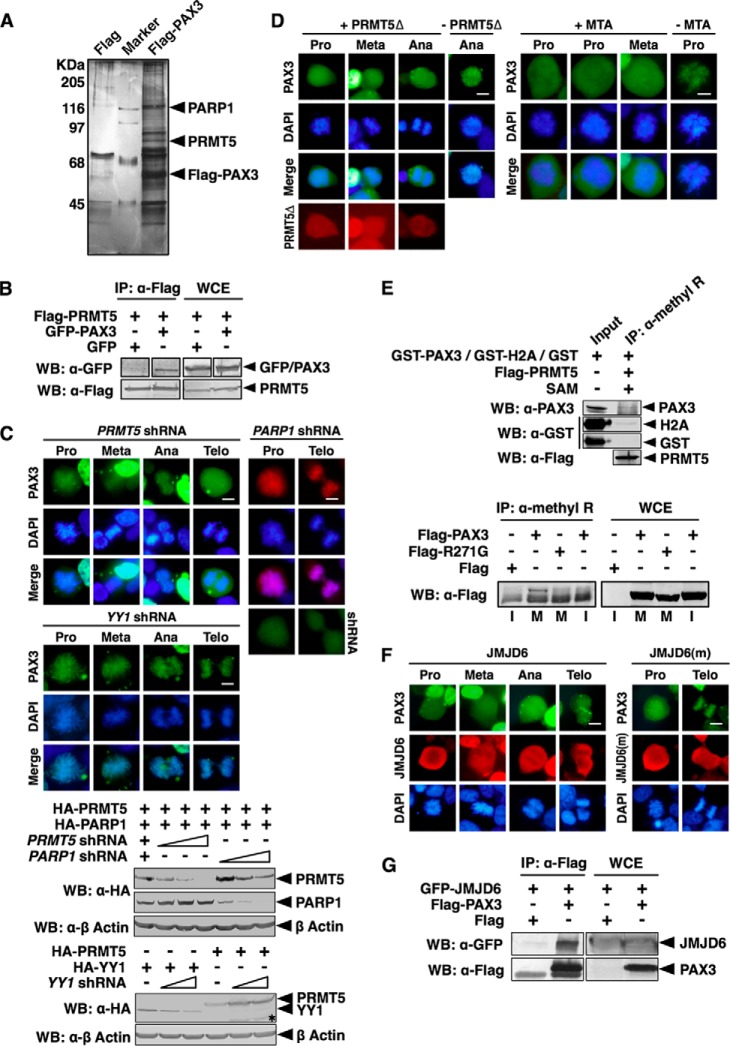
FIGURE 3.
Loading of PAX3 on mitotic chromosomes required arginine methyltransferase activity of PRMT5. A, PRMT5 is a component of the PAX3 protein complex. A FLAG-PAX3 protein complex was purified from HEK 293 cells as described under “Experimental Procedures.” Components of the PAX3 protein complex were separated by SDS-PAGE, visualized by silver staining, and identified by LC-MS/MS. B, PAX3 interacts with PRMT5 in a coimmunoprecipitation assay. + indicates the plasmids transfected into HEK 293 cells. Whole cell extracts (WCE) and anti-FLAG immunoprecipitates (IP: α-FLAG) were immunoblotted by anti-FLAG and anti-GFP as indicated. WB, Western blot. C, knocking down PRMT5 prevents localization of PAX3 to the mitotic chromosome. PRMT5 or YY1 shRNA was transfected into HEK 293 cells with GFP-PAX3 as indicated. Mitotic chromosome localization of GFP-PAX3 was determined by fluorescence microscopy. Cells expressing PARP1 shRNA and FLAG-PAX3 served as the control, and the expression of the PARP1 shRNA was confirmed by the fluorescence from the GFP tag of the shRNA construct (right panels). Knockdown efficiency of shRNAs was determined by Western blotting. An asterisk indicates nonspecific protein bands. Meta, metaphase; Ana, anaphase; Telo, telophase. D, inhibition of the methyltransferase activity of PRMT5 prevents PAX3 from binding to the mitotic chromosome. Left panels, FLAG-PRMT5Δ (a PRMT5 dominant negative mutant) and GFP-PAX3 were transfected into HEK 293 cells. Mitotic chromosome localization of GFP-PAX3 was determined by fluorescence microscopy. Right panels, HEK 293 cells expressing GFP-PAX3 were treated with the PRMT enzyme inhibitor methylthioadenosine (MTA) for 24 h before analysis of localization of PAX3. E, PAX3 can be detected by an anti-methylarginine antibody specifically during mitosis. Upper panel, in vitro methylation reactions containing GST fusion proteins (GST-PAX3, GST-H2A, or GST) purified from HEK 293 cells, FLAG-PRMT5 (also purified from HEK 293 cells), and S-(5′-adenosyl)-l-methionine (SAM) were immunoprecipitated with an anti-methylarginine antibody and then blotted with anti-PAX3, anti-GST, or anti-FLAG antibodies. Lower panel, HEK 293 cells were transfected with expression vectors indicated and then synchronized. Anti-methylarginine immunoprecipitates from transfected cells synchronized at mitotic phase (M) or interphase (I) was blotted with the anti-FLAG antibody. FLAG-R271G, FLAG-tagged PAX3 with the R271G mutation in HD. F, arginine demethylase JMJD6 pulls PAX3 from mitotic chromosomes. FLAG-JMJD6 or FLAG-JMJD6(m) (a mutant JMJD6 without enzyme activity) was transfected with GFP-PAX3 into HEK 293 cells. Mitotic chromosome localization of GFP-PAX3 was determined by fluorescence microscopy. G, JMJD6 interacted with PAX3. HEK 293 cells were transfected with plasmids as indicated. Anti-FLAG immunoprecipitates (α-FLAG) and whole cell extracts were immunoblotted with anti-FLAG and anti-GFP antibodies as indicated.