FIGURE 6.
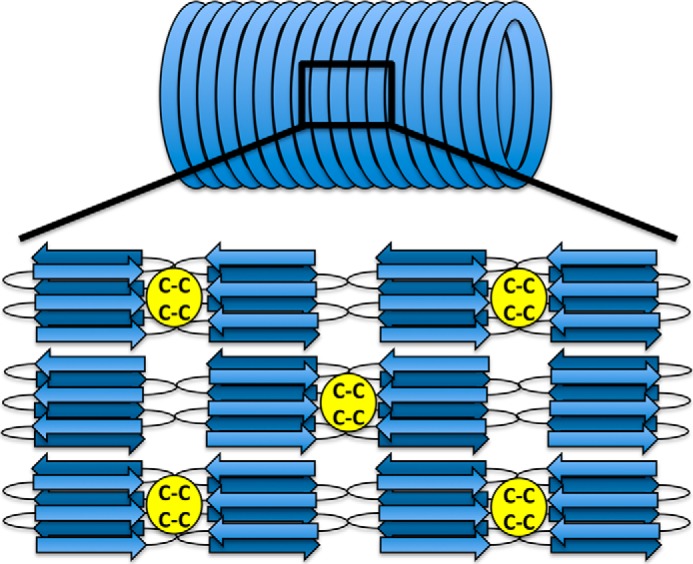
A hypothetical model of the organization of MspA molecules within the sheath. The structure is based on our data and previous electron diffraction data (13). Two closely spaced cysteine residues found within the middle of the MspA sequence may allow for the formation of intermolecular disulfide bonds that links the hoops together. The linkage could either be direct or mediated by small linker peptides. The exact number of β-strands formed by each MspA subunit is not known.
