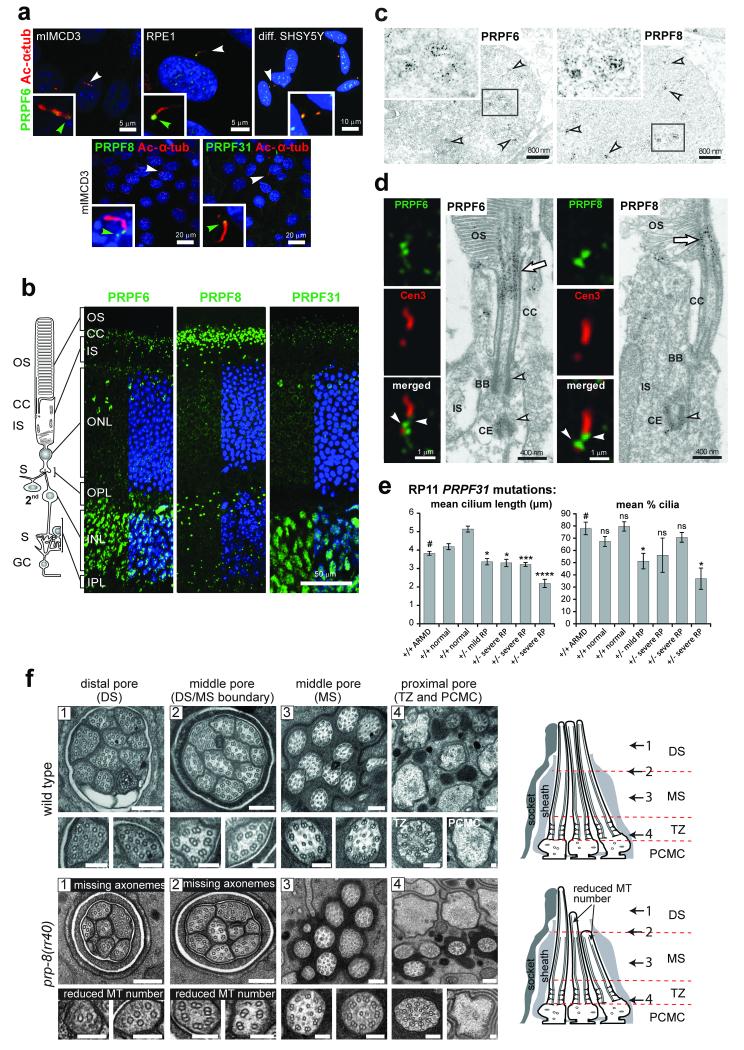
Figure 4. Ciliary localisation and functional effect on ciliary axonemal formation of pre-mRNA processing factors.
(a) PRPF6, PRPF8 and PRPF31 (green) localise to proximal/basal regions of primary cilia (green arrowheads) and nuclear speckles in the indicated cell-lines. Selected cells (white arrowheads) are magnified (insets). (b) PRPF6, PRPF8 and PRPF31 (green) localise to the ciliary regions of photoreceptor cells (connecting cilium, CC) and nuclei of inner nuclear layer (INL) in longitudinal sections of adult murine retinas. Retinal layers are depicted schematically: photoreceptor outer segment (OS), connecting cilium (CC), and inner segment (IS); secondary neurons (2nd); ganglion cell layer (GC); synaptic region (S) in the outer and inner plexiform layer (OPL and IPL). (c) Immunoelectron microscopy confirms localisation of PRPF6 and PRPF8 in nuclear speckles in the INL (arrowheads). Frames indicate the magnified insets. (d) Immunofluorescence of PRPFs (green) and the ciliary marker centrin-3 (red), and immunoelectron microscopy, reveal localisation of PRPF6 and PRPF8 at the basal body (BB) and adjacent centriole (CE) of the CC (arrowheads) and apical CC (arrow). (e) Primary cilia length and number measurements for dermal fibroblasts from normal healthy controls, age-matched disease-control (ARMD), and four patients with RP type 11 of variable severity carrying heterozygous (+/−) PRPF31 frame-shift mutation c.1115_1125delGAAGCAGGCCA. Significance of pair-wise comparisons with the disease negative control (#): ns, not significant; * p<0.05, *** p<0.001, **** p<0.0001 (paired two-tailed Student’s t-test), for n=4 independent experiments. Error bars indicate s.d. (f) Transmission EM images of sensory cilia from sequential cross sections (numbered arrows in schematics) of C. elegans amphid pore in wild-type (N2) and prp-8(rr40) mutants. Wild-type amphid pores contain 10 ciliary axonemes, each consisting of a distal segment (DS; singlet A microtubules), middle segment (MS; doublet A/B microtubules), transition zone (TZ) and periciliary membrane compartment (PCMC). In prp-8(rr40) mutants, axonemes are missing in DS and DS/MS boundary, and microtubule (MT) number is reduced. Images are representative of four analysed amphid pores for each strain. Scale bars: (a) 20 μm and 5 μm; (b) 50 μm; (c) 800 nm; (d) 1 μm and 0.4 μm; (f) 200 nm (low magnification images), 100 nm (high magnification images).