Figure 2. Role of c-Src in migration and invasion properties of MDA-MB-231-Tet-On-shRNA-c-Src cells.
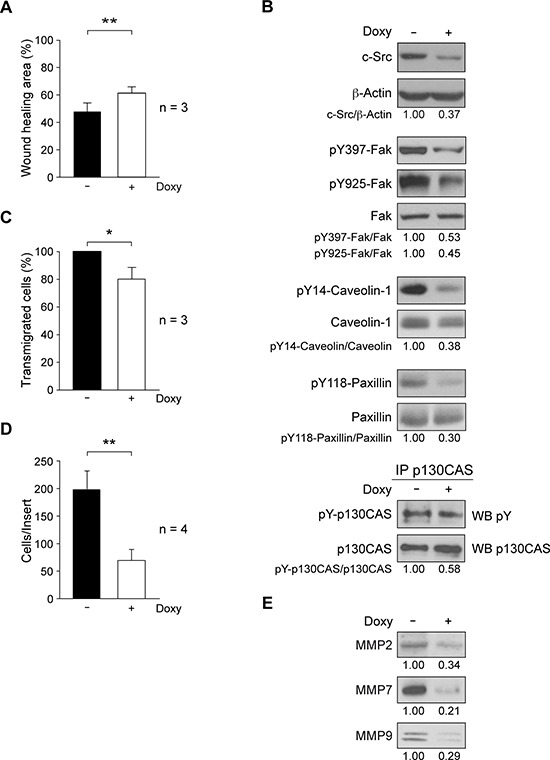
A. Cell migration was determined by wound-healing assay through scratching confluent cultures; photomicrographs were taken at 0 and 20 h with a Microscope Cell Observer Z1 system, and quantified using wound-healing tool of ImageJ. Results are expressed as mean percentage of wound healing area ± SD at 20 h respect to 0 h from three independent experiments (**p < 0.01). B. Expression of phosphoproteins/proteins involved in cell motility by immunoblotting. Extracts from control and treated cells (2 μg/ml Doxy, 72 h) were blotted with antibodies to c-Src (MAb-327), pY397-Fak, pY925-Fak, pY14-Caveolin and pY118-Paxillin. p130CAS was immunoprecipitated from total cell extracts and immune-complexes blotted with anti-pY (4G10). Membranes were reblotted with anti-β-actin (for c-Src) and anti-total-protein (for phosphoproteins) for loading control. Results are representative of three independent experiments. C. Transendothelial migration through a HUVEC monolayer. Cells were grown for 48 h −/+ 2 μg/ml Doxy and then seeded on the HUVEC monolayer. Transmigrated cells were detached after 22 h and counted in a hemocytometer. The number of Doxy-treated transmigrated cells was expressed as percentage of control transmigrated cells (100%). Assay was repeated three times (*p < 0.05). D. For cell invasion through Matrigel-coated inserts, cultures were grown for 48 h −/+ 2 μg/ml Doxy and then seeded onto Matrigel (−/+ 2 μg/ml Doxy); 22 h later, cells on the top of inserts were removed and invaded cells were fixed, stained with DAPI and counted by fluorescence microcopy. The number of invaded cells per insert is shown and represents average ± SD of four experiments in triplicate (**p < 0.01). E. Analysis of secreted metalloproteinases MMP2, MMP7 and MMP9 from equal number of control or Doxy-treated cells (2 μg/ml) for 72 h. Conditioned media were used to prepare total soluble fraction of secretome by differential centrifugation (S3, Figure 4A). After concentration by methanol/chloroform precipitation, pellet was resuspended in RIPA for immunoblotting analyses (see Material and Methods). Results are representative of three independent experiments.
