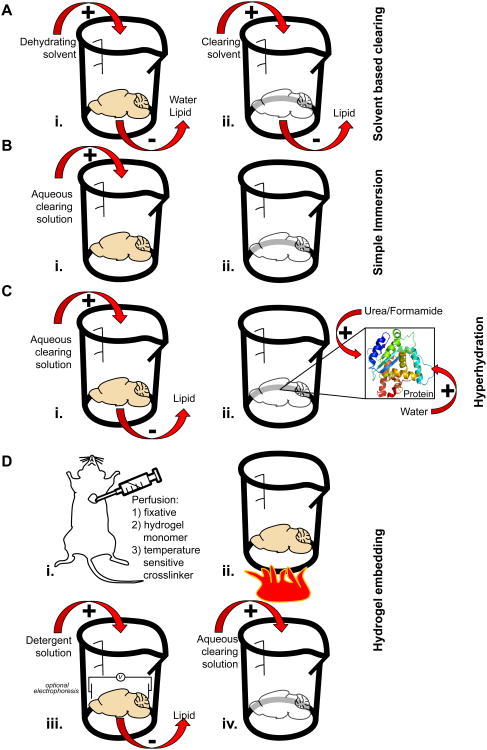
Figure 2. Methodology of tissue clearing techniques.
(A) Left, Solvent based clearing is a two-step process. First, the tissue is dehydrated and lipid is removed. Second, the tissue is moved to a high refractive index solvent where additional lipid solvation and clearing occurs. Right, Molecules commonly used for solvent based clearing along with the refractive indices (RI) of the pure chemical. (B) Left, For simple immersion, the tissue to be cleared is placed in an aqueous clearing solution for days to months. During this time the solution is exchanged repeatedly. Right, Molecules commonly used for simple immersion along with the refractive indices (RI) at the commonly used concentration. (C) Hyperhydration involves submerging the sample in an aqueous solution and allowing it to passively clear. During this clearing step, urea or formamide in the clearing solution can enter tightly folded regions of high refractive index proteins creating an osmotic gradient that pulls in water as well. This partially denatures the protein, hydrates it and decreases its overall refractive index. Some hyperhydration methods contain detergent which is used to disrupt membranes and remove lipid from the sample. (D) Left, Hydrogel embedding is most often performed on an entire animal by perfusing with a fixative, a temperature sensitive crosslinker, and the hydrogel monomer. Alternatively, these chemicals can be passively diffused into an isolated tissue sample. Once fixed, the tissue of interest is warmed to induce hydrogel crosslinking. The sample is then placed in a detergent solution to remove lipid material passively or via an electrophoretic charge. Finally the lipid-free sample is placed in a high-refractive index matching solution for clearing. Histodenz is one high refractive index molecule that can be a component of this clearing solution. Glycerol, TDE or Diatrizoic acid also can play this role.