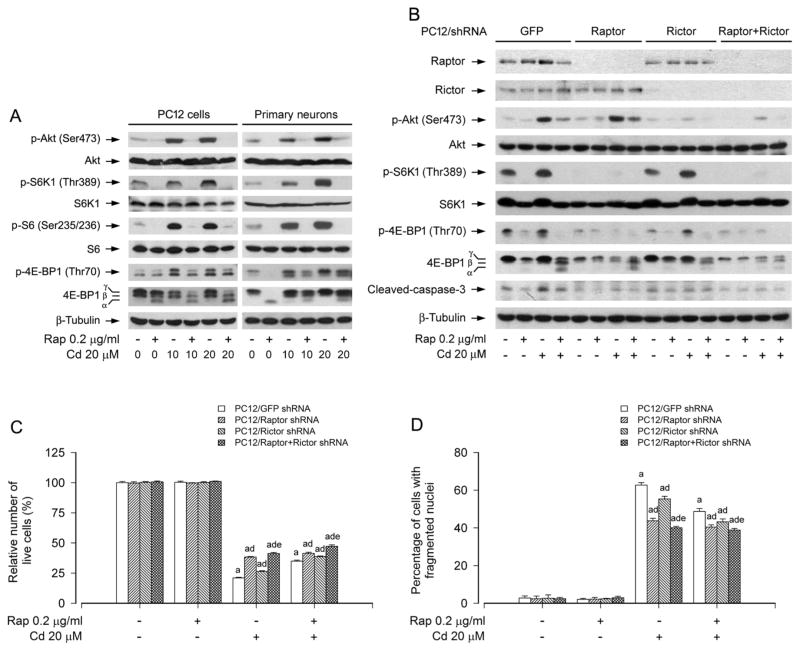
Fig. 3.
mTORC1 and mTORC2 are involved in rapamycin’s prevention from Cd-induced neuronal cell death. PC12 cells and primary neurons, or PC12 cells infected with lentiviral shRNAs to raptor, rictor, raptor/rictor or GFP (as control), were pretreated with rapamycin (Rap, 0.2 μg/ml) for 48 h, followed by exposure to Cd (10 and/or 20 μM) for 4 h (for Western blotting) or 24 h (for live cell assay and DAPI staining). A) Rapamycin strongly blocked Cd-induced phosphorylation of Akt, S6K1 and 4E-BP1 in PC12 cells and primary neurons. B) Lentiviral shRNA to raptor, rictor or raptor/rictor, but not GFP, downregulated raptor, rictor or raptor/rictor in PC12 cells, respectively. For A) and B), the blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. C and D) Silencing raptor, rictor or raptor/rictor potentiated rapamycin resistance to Cd-induced C) cell viability reduction and D) apoptosis, and this effect was more potent in the double raptor/rictor-silenced cells than in the single raptor or rictor-silenced cells. Results are presented as mean ± SEM (n = 5). a p < 0.05, difference with control group; b p < 0.05, difference with 10 μM Cd group; c p < 0.05, difference with 20 μM Cd group. d p < 0.05, Raptor shRNA group, Rictor shRNA group or Raptor/Rictor shRNA group versus GFP shRNA group; e p < 0.05, Raptor/Rictor shRNA group versus Raptor shRNA group or Rictor shRNA group.