Abstract
Exposures to various types of early life stress can be robust predictors of the development of psychiatric disorders, including depression and anxiety. The objective of the current study was to investigate the roles of the translationally relevant targets of central vasopressin, oxytocin, ghrelin, orexin, glucocorticoid, and the brain-derived neurotrophic factor (BDNF) pathway in an early chronic social stress (ECSS )based rodent model of postpartum depression and anxiety. The present study reports novel changes in gene expression and extracellular signal related kinase (ERK) protein levels in the brains of ECSS exposed rat dams that display previously reported depressed maternal care and increased maternal anxiety. Decreases in oxytocin, orexin, and ERK proteins, increases in ghrelin receptor, glucocorticoid and mineralocorticoid receptor mRNA levels, and bidirectional changes in vasopressin underscore related work on the adverse long-term effects of early life stress on neural activity and plasticity, maternal behavior, responses to stress, and depression and anxiety-related behavior. The differences in gene and protein expression and robust correlations between expression and maternal care and anxiety support increased focus on these targets in animal and clinical studies of the adverse effects of early life stress, especially those focusing on depression and anxiety in mothers and the transgenerational effects of these disorders on offspring.
Keywords: early life stress, depression, anxiety, postpartum depression, oxytocin, vasopressin, ghrelin, orexin, mineralocorticoid receptor, neuroplasticity
Introduction
Exposures to various types of early life stress can be robust predictors of the development of psychiatric disorders, including depression and anxiety (Eiland and McEwen, 2012; Heim and Binder, 2012; Heim and Nemeroff, 2001; Heim et al., 1997; Johnson and Sarason, 1978; McEwen, 1998; McEwen, 2003). Adverse family social environments are strongly associated with the development of depression (Bouma et al., 2008; Essex et al., 2011; Lizardi et al., 1995) and postnatal exposure to maternal depression has negative effects on offspring mental health (Essex et al., 2011; Goodman et al., 2011). It is postulated that maternal depression exerts its adverse influence through impaired mother-infant bonding (Bureau et al., 2009; Gunnar and Vazquez, 2006; Milan et al., 2009).
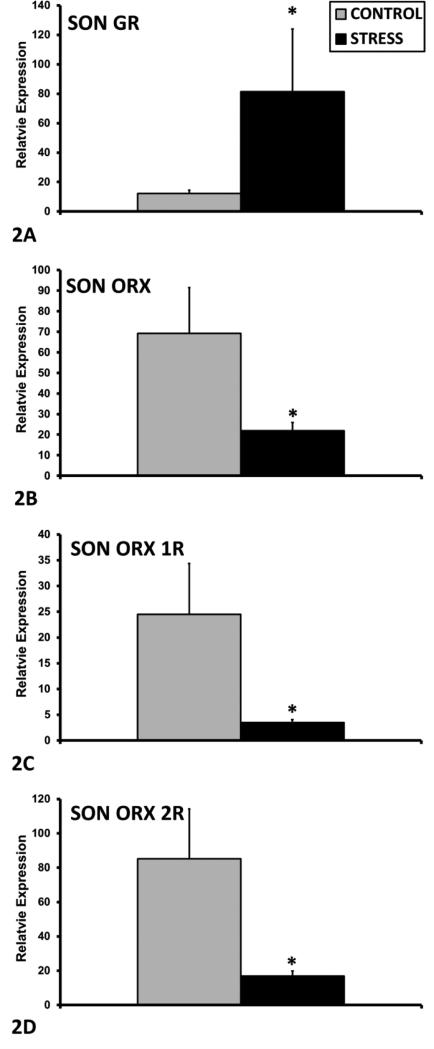
In rodent dams, chronic social stress (CSS, daily exposure to a novel male intruder) can be used as an ethologically relevant, transgenerational model of the role of stress in the etiology of depression and anxiety in mothers and their offspring (Babb et al., 2014; Carini et al., 2013; Carini and Nephew, 2013; Murgatroyd and Nephew, 2013; Nephew and Bridges, 2011)(see figure 1). Exposure of F0 lactating dams to CSS as a model for postpartum depression and anxiety possesses construct and face validity and depresses maternal care and increases anxiety (Carini et al., 2013; Nephew and Bridges, 2011). For the young F1 offspring of stressed F0 dams, CSS is a robust early chronic social stress (ECSS) which includes exposure to both the depressed maternal care from their F0 mothers and the conflict between the F0 dam and the male intruders. Similar to observations in human mothers exposed to high levels of early life stress (Goodman, 2007), the maternal care displayed by F1 dams towards their F2 offspring is also depressed (Carini and Nephew, 2013; Murgatroyd and Nephew, 2013). Furthermore, the social behavior of both male and female F2 offspring (exposed to depressed maternal care from their F1 mothers) is impaired (Babb et al., 2014). Since maternal depression can often be predicted from an exposure to early life stress, the CSS F1 and F2 generations represent relevant models to study the role of ECSS in postpartum depression and anxiety and the adverse effects of these disorders on offspring. Peripheral and central endocrine studies of the CSS model reveal substantial changes in the behaviorally relevant hormones oxytocin (OXT), vasopressin (AVP), prolactin (PRL), estradiol, and corticosterone (Carini and Nephew, 2013; Murgatroyd and Nephew, 2013). In the brain, OXT, AVP, and PRL gene expression are altered in the hypothalamus of ECSS exposed dams (Murgatroyd and Nephew, 2013). OXT, AVP, and PRL are primary mediators of maternal care and have been implicated in the etiology and symptomology of stress related affective disorders (Faron-Górecka et al., 2014; Insel and Young, 2001; Mann and Bridges, 2001; Nephew, 2012; Rilling and Young, 2014; Zamorano et al., 2014). Furthermore, OXT is a key mediator of the reciprocal nature of the mother-infant bond (Carter, 2003; Feldman et al., 2011; Feldman et al., 2007; Henriques et al., 2014; MacKinnon et al., 2014; Mogi et al., 2011). The current study investigated additional neural targets that may be involved in the adverse effects of ECSS on the F1 generation and represent novel preventative and/or treatment targets.
Figure 1.
Diagram of the Chronic Social Stress paradigm. The current study involved the dams from the F1 generation. Testing on postpartum days 2, 9, and 16 included maternal care and maternal aggression. Brain samples from the F1 dams were obtained on postpartum day 23 when the F2 pups were weaned. These samples were analyzed for the expression of oxytocin, oxytocin receptor, vasopression, vasopression V1a receptor, prolactin receptor, glucocorticoid and mineralocorticoid receptors, orexin A, orexin receptors 1 and 2, ghrelin receptor, and protein levels of BDNF, ERK1/2, and phospho-ERK1/2.
The maladaptive impacts of early life stress on mental health are mediated in part through changes the hypothalamic–pituitary–adrenal (HPA) axis (Gunnar and Vazquez, 2006), although there is growing support for the involvement of multiple interacting brain regions and neuroendocrine factors (Lucassen et al., 2014). There is strong evidence that early life stress has persistent effects on the regulation of the HPA axis through altered gene expression in the brain which involves changes in glucocorticoid receptors (GR) (Liu, 1997; Lupien et al., 2009; McGowan et al., 2009; Suderman et al., 2012) as well as more recent data on mineralocorticoid receptors (MR) (Baes et al., 2014; Juruena et al., 2013; Klok et al., 2011a; Young et al., 2003). It has been suggested that MR receptor activity is increased in patients with depression compared to controls, and a systematic review of the role of GR and MR in early life stress related depression concludes that the effects of stress on the GR/MR ratio may be a key etiological factor in depression, although there are few clinical studies that have investigated this role (Von Werne Baes et al., 2012). Given the previously reported HPA changes in the ECSS exposed F1 dams, it was postulated that changes in both MR and GR may mediate these effects.
Recent studies suggest that ghrelin, an orexigenic hormone, may be a primary mediator of the adverse effects of stress on behavior (Ishitobi et al., 2012; Lutter et al., 2008). Although ghrelin is produced in the stomach, it crosses the blood brain barrier (Banks et al., 2002) and ghrelin receptors (GHR) have been found in several brain regions, including the paraventricular nucleus (PVN), amygdala, and ventral tegmental area (VTA) (Alvarez-Crespo et al., 2012; Perello et al., 2012). A Leu72Met polymorphism in the ghrelin gene coding region associates with depression (Nakashima et al., 2008), and elevated ghrelin levels have been found in patients with treatment-resistant depression (Ishitobi et al., 2012), suggesting that ghrelin may be a useful indicator of treatment efficacy. In addition, the orexin system, including the expression of orexin and its receptors (Ox1R and Ox2R) has been implicated in the expression of both maternal care (D'Anna and Gammie, 2006) and depressive behavior and pathophysiology (Arendt et al., 2013; Nollet and Leman, 2013). The clinical and rodent studies indicate that ghrelin and orexin may mediate the depressed maternal care in dams exposed to ECSS.
The brain-derived neurotrophic factor (BDNF) pathway, including extracellular signal regulated kinase (ERK) signaling, is another mechanistic target for depression research. In a rat model of infant maltreatment, decreased BDNF levels were found to be programmed through an epigenetic mechanism (Roth et al., 2009). In mice, the inhibition of ERK signaling in hippocampus induces depression-like behavior and blocks the behavioral effects of antidepressants (Duman et al., 2007; Schmidt and Duman, 2007). Adult rodent exposure to exogenous corticosterone affects phospho-ERK1/2 levels in the dentate gyrus, and these effects are sensitive to antidepressant treatment (Gourley et al., 2008). Long-term changes in the BDNF pathway are associated with childhood adversity and adult depression symptoms (Aguilera et al., 2009; Gatt et al., 2009). Mitogen-activated protein kinase phosphatase-1 (MKP-1) expression is increased in the postmortem hippocampus of patients with major depression compared to healthy controls, and increasing MKP-1 activity in rodent models induces depressive behaviors (Duric et al., 2010) and is further regulated by BDNF (Jeanneteau et al., 2010). In the transgenerational CSS model, we propose that ECSS-induced decreases in the BDNF pathway in a nucleus involved in the processing of rewarding stimuli, the nucleus accumbens, may mediate decreased maternal care and increased anxiety.
The objective of the current study was to augment the previous investigation of the endocrine and behavioral effects of ECSS in the F1 generation of CSS model of depression and anxiety (Carini and Nephew, 2013) with the addition of neuroendocrine analyses of vasopressin, oxytocin, prolactin, ghrelin, orexin, corticosteroid receptors, and ERK pathways. These targets are both translationally and clinically relevant. This broad, yet targeted, approach was taken because complex behaviors including maternal care are dependent upon multiple interacting brain regions, and ECSS may interact with each of these structures in distinct, meaningful ways. ECSS-induced changes in peripheral estrogen, PRL, corticosterone, maternal behavior, and lactation in the F1 dams in this study have been previously reported (Carini and Nephew, 2013). It is hypothesized that AVP, OXT, PRL, orexin and BDNF related gene expression and/or protein levels will be down regulated in key brain regions in ECSS F1 dams, ghrelin receptors, GR and MR will be up-regulated, and these changes will be associated with impaired maternal behavior.
METHODS
Animals
Animals in this study were maintained in accordance with the guidelines of the Committee of the Care and Use of Laboratory Animals Resources, National Research Council, and the research protocol was approved by the Tufts Institutional Animal Care and Use Committee. “CSS dams” refers to the adult females exposed to CSS during lactation (F0), and “ECSS dams” refers to the adult female offspring of the CSS dams (F1); the focus of the present study. All the neuroendocrine data and behavioral correlations are from ECSS dams (fig. 1).
CSS model: creation of F0 dams
Dams (Charles River, Wilmington, MA) mated at Tufts University were subjected to the CSS protocol (previously described by Nephew and Bridges, 2011) consisting of placing a similarly sized (220–300 g) novel male intruder into a lactating female's home cage for 1h from days 2 to 16 of lactation. Control dams were not exposed to the CSS protocol, and were only tested for maternal care and maternal aggression between 0800 and 1200 on days 2, 9, and 16 of lactation (both control and CSS dams were tested for maternal care and maternal aggression on these days). The F1 pups were left in the cage during the intruder presentation and the F1 CSS pups were exposed to depressed maternal care from their F0 mothers and the daily conflict between the mother and the male intruder (Nephew and Bridges, 2011). The rationale for the weekly testing was to avoid introducing potential confounds involved with an excessive amount of dam/pup separations into the model.
ECSS: creation of F1 females
The control and ECSS F1 females of the current study were the offspring of the F0 control and CSS dams; the differences between the treatments of the control and ECSS F1 females were limited to the exposure of the ECSS F1 females to depressed maternal care and daily conflict between their F0 mothers and the male intruders during age 2 to 16 days. The F1 control and ECSS animals were treated identically after the age of 16 days. After weaning all F1 pups on day 23, the female F1 offspring from the twelve F0 control and twelve CSS dams were housed in groups of four until 70 days of age when two from each litter were mated with 6 proven breeder males in groups of 12 (18 F1 females for both the control and ECSS groups). Initial samples sizes for the F1 dams were 14, but 2 control dams were removed from the study due to small litter sizes (5 and 6), resulting in n's of 12 for the control, and 14 for the F1 ECSS group. Total F2 pup number and litter weights were recorded on the day of parturition, and litters were then culled to five females and five males. There were no group differences in the dam weights or number or weights of pups at birth and across lactation (Carini and Nephew, 2013). Total maternal care was defined as the cumulative duration of pup grooming and nursing, and maternal anxiety was defined as the combined duration of self grooming, nesting, and non-maternal locomotion during a 30 minute maternal care observation on days 2, 9, and 16 between 0800 and 1200, the same testing protocol used with F0 maternal care testing. We have previously reported depressed maternal care and/or elevated maternal anxiety in the current ECSS dams throughout lactation, with the most substantial effects on day 2 of lactation and an overall decrease in total maternal care over all three days of testing in the F1 CSS group compared to controls (Carini and Nephew, 2013). The decrease in maternal care was due to significant decreases in the durations of both pup grooming and nursing. While ECSS caused an overall decrease in maternal care throughout lactation (days 2, 9, and 16), the greatest effects on both maternal care and maternal anxiety were during early lactation on day 2 (Carini and Nephew, 2013), a critical period for the effects of maternal care on offspring gene expression and development (Champagne et al., 2003; Peña et al., 2013). All F1 dams were euthanized on day 23 of lactation and the brains were extracted and stored at −80C. The final sample sizes at the end of lactation were 12 F1 control dams and 14 ECSS dams.
RNA expression analyses
Total RNA and DNA were simultaneously extracted from paraventricular nucleus (PVN), supraoptic nucleus (SON), medial amygdala (MeA), and central amygdala (CeA) brain punches (Bettscheider et al., 2011) and reverse transcription (RT) reactions (Bioline) were performed on 200 ng RNA using random primers to analyze transcript levels. Quantitative PCR (qPCR) was performed on a StepOne Plus (Applied Biosystems) using Sensi Fast SYBR Green (Bioline). Primer sequences and conditions for qPCR reactions are listed in Table 1. Expression levels for OXT, OXT receptor (OXT R), AVP, AVP V1a receptor (AVPR), the long form of the PRL receptor (PRL R), GR (Nr3c1), MR (Nr3c2), Orx1r, Orx2r, Orexin A, and Ghrelin R were normalized against three combined housekeeping genes, β -actin, hypoxanthine phosphoribosyltransferase (Hprt) and glyceraldehyde-3-phosphate dehydrogenase (Gapdh).
TABLE 1.
PRIMERS
| Gene | Primer sequences |
|---|---|
| AVP | F-CAGATGCTCGGCCCGAAG; R-TTCCAGAACTGCCCAAGAG |
| AVPR | F-CAGCAGCGTGAAGAGCATTT; R-CGCCGTGATTGTGATGGAAG, |
| OXT | F-TCTGACCTCCGCCTGCTACATC; R-AAGCAGCGCCTTTGCCGCC, |
| OXTR | F-GTACTGGCCTTCATCGTGTGC; R-TGCAGCAGCTGTTGAGGCTG, |
| PRLR | F-GTAGATGGAGCCAGGAGAGTTC; R-ACCAGAGTCACTGTCGGGATCT |
| GR (Nr3c1) | F-AGGGGAGGGGGAGCGTAATGG; R-CCTCTGCTGCTTGGAATCTGC, |
| MR (Nr3c2) | F-TACGACAATTCCAAGCCCGACACC; R-TACCTTGGCCCACTTCACGACCTG |
| Orx1r | F-AGGTGGATGGAAGCGTGAAG; R-AGAGATAATCGCGCCACAGG, F- |
| Orx2r | F-CGCAACTGGTCATCTGCTTC; R-TTCGTGCTCGGATCTGCTTT |
| Orexin A | F-CCACTGCACCGAAGATACCA; R-AGTTCGTAGAGACGGCAGGA |
| Grehlin R | F-TGTGGGTGTCCAGCGTCTT; R-GCAGAGGATGAAAGCAAACACC |
| B-actin | F-TTGCTGACAGGATGCAGAA; R-ACCAATCCACACAGAGTACTT, |
| Hprt | F-TGGTCAAGCAGTACAGCCCC; R-TACTGGCCACATCAACAGGA; |
| Gapdh | F-CATCACCATCTTCCAGGAGC; R-TAAGCAGTTGGTGGTGCAGG, |
Immunoblotting
Protein levels from brain punches of the NAc were analyzed as described previously (Krishnan et al., 2007b). Briefly, samples were homogenized by light sonication in RIPA buffer containing protease and phosphatase inhibitors. Proteins were separated on 4-15% polyacrylamide gradient gels (Criterion System, BioRad), and analyzed by western blotting with the antibodies indicated. Quantification of bands was analyzed by normalizing to corresponding beta-tubulin levels, and phospho-ERK was normalized to total ERK (Image J). Primary antibodies used were against AKT (Cell Signaling 4691; 1:1000), BDNF (Santa Cruz SC-546, 1:500), beta-tubulin (Cell Signaling 2128, 1:1000), ERK1/2 (p44/42 MAPK, Cell Signaling 4695, 1:1000), phospho-ERK1/2 (p44/42 MAPK, Cell Signaling 4370, 1:2000), FosB (Santa Cruz SC-48, 1:500).
Statistics
Relative mRNA expression and protein levels were compared with individual ANOVA for each brain region. Where non-significant trends in the ANOVA results were present, these tests were followed with 1-tailed t-tests with Benjamini and Hochberg multiple comparison correction (Benjamini and Hochberg, 1995) if justified by previous studies of the CSS model (OXT and GR). We have previously reported decreased OXT in the MeA (Murgatroyd and Nephew, 2013), and have observed a significant increase in hypothalamic GR expression in the F0 dams which is associated with decreased methylation at the CpG2 promoter region (data submitted for publication). Pearson correlations were used to test for significant gene-behavior associations in restricted data sets (total maternal care and total maternal anxiety on lactation day 2 with the 12 significant differences in gene expression/protein levels (figs. 2-5) in the control and ECSS groups, and both groups combined). All graphical results are presented as mean + SEM, and the level of statistical significance was p < 0.05.
Figure 2.
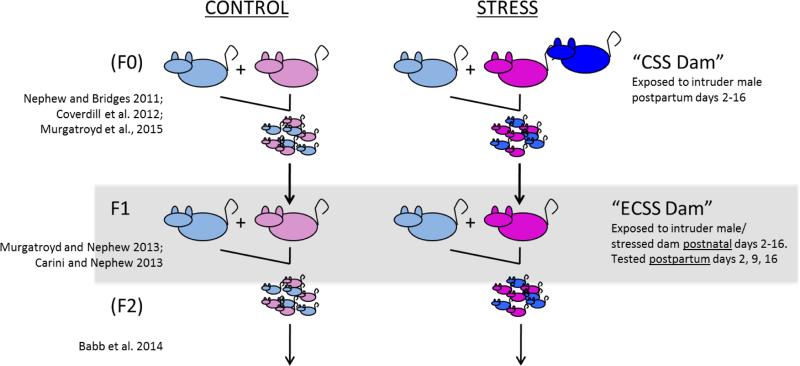
Mean + SEM relative mRNA expression levels of AVP (A), Ghrelin R (B), MR (C), and GR/MR ratio (D) in the PVN of control (n=12) and ECSS (stress) (n=14) dams. * Indicates a significant effect of CSS, p<0.05
Figure 5.
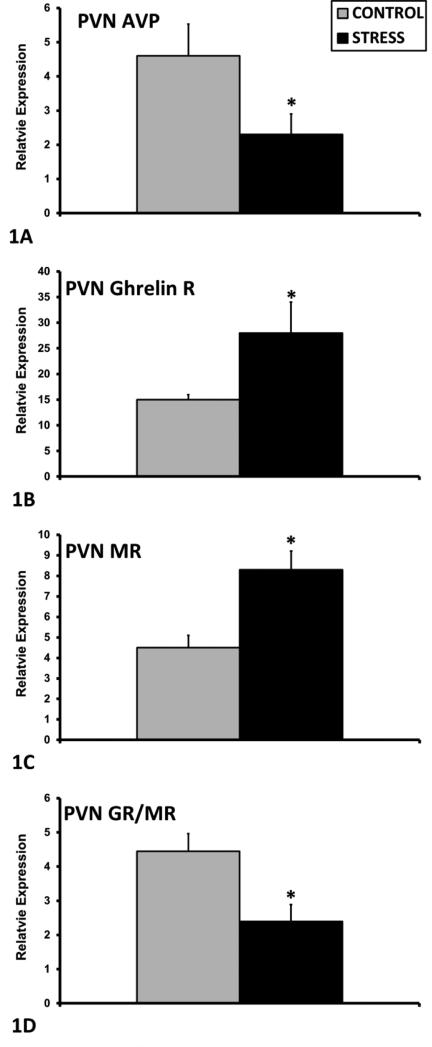
Mean + SEM relative protein levels (normalized to beta-tubulin) of total ERK (A) and phospho-ERK/total ERK ratio (B) in the NAc of control (n=12) and ECSS (stress) (n=14) dams. * Indicates a significant effect of CSS, p<0.05
RESULTS
Gene and Protein Expression
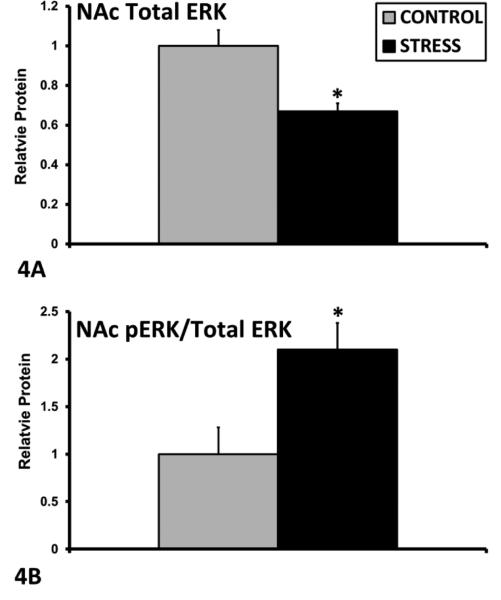
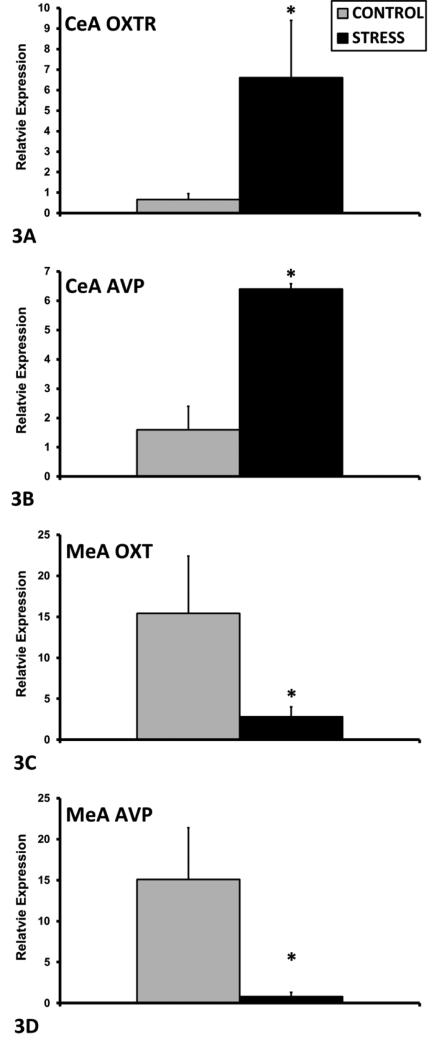
In the PVN, exposure to ECSS was associated with decreased AVP mRNA expression among F1 dams (F1,25= 4.1, p=0.05, Fig. 1A), and increased Ghrelin R (F1,25= 5.8, p<0.05, Fig. 1B) and MR (F1,25= 12.4, p<0.01, Fig. 1C) mRNA. In addition, the GR/MR mRNA ratio was decreased in the ECSS dams (F1,25= 8.3, p<0.01, Fig. 1D). In the SON, GR expression was increased in ECSS dams (F1,25= 3.0, p=0.1, t<0.05 Fig. 2A), and Orexin A (F1,25= 4.7, p<0.05, Fig. 2B), Orx1R (F1,25= 4.9, p<0.05, Fig. 2C) and Orx2R (F1,25= 6.4, p<0.05 Fig. 2D) were all decreased in stressed dams. In the CeA, ECSS was associated with increased OXTR (F1,25= 6.1, p<0.05, Fig. 3A) and AVP (F1,25= 5.7, p<0.05, Fig. 3B) mRNA. In the MeA, expression of both OXT (F1,25= 3.7, p=0.07, t=0.03, Fig. 3C) and AVP (F1,25= 5.0, p<0.05, Fig. 3D) were decreased. Investigation of BDNF and ERK protein levels in the NAc revealed decreased total ERK protein (F1,25= 13.7, p<0.01, Fig. 4A) but an elevated phosphorylated ERK/total ERK ratio (F1,25= 7.7, p<0.01, Fig. 4B), with a trend for elevated pERK relative to beta-tubulin.
Figure 3.
Mean + SEM relative mRNA expression levels of GR (A), Orexin (B), Orx1R (C), and Orx2R (D) in the SON of control (n=12) and ECSS (stress) (n=14) dams. * Indicates a significant effect of CSS, p<0.05
Figure 4.
Mean + SEM relative mRNA expression levels of OXTR (A) and AVP (B) in the CeA and of OXT (C), and AVP (D) in the MeA of control (n=12) and ECSS (stress) (n=14) dams. * Indicates a significant effect of CSS, p<0.05
Gene-Behavior Correlations (Table 2)
TABLE 2.
Gene Expression : Maternal Behavior Correlations
| Gene | Nucleus | Day 2 Behavior | Group | r | R2 | P value |
|---|---|---|---|---|---|---|
| AVP | CeA | Maternal Anxiety | ELSS | −0.67 | 0.44 | 0.03 |
| AVP | MeA | Maternal Anxiety | ELSS | 0.58 | 0.34 | 0.03 |
| AVP | PVN | Maternal Care | CON+ELSS | 0.51 | 0.26 | 0.01 |
| AVP | PVN | Maternal Anxiety | CON+ELSS | −0.46 | 0.21 | 0.02 |
| ERK | NAc | Maternal Care | CON+ELSS | 0.76 | 0.57 | <0.001 |
| ERK | NAc | Maternal Anxiety | CON+ELSS | −0.64 | 0.41 | <0.001 |
| GHR | PVN | Maternal Anxiety | CON+ELSS | 0.55 | 0.31 | 0.003 |
| GR | SON | Maternal Care | CON | −0.53 | 0.28 | 0.04 |
| GR | SON | Maternal Anxiety | ELSS | −0.58 | 0.34 | <0.05 |
| MR | PVN | Maternal Anxiety | CON+ELSS | 0.51 | 0.26 | 0.01 |
| Orexin A | SON | Maternal Care | CON | −0.66 | 0.44 | 0.02 |
| Orx1R | SON | Maternal Care | CON | −0.60 | 0.36 | 0.04 |
| Orx1R | SON | Maternal Anxiety | ELSS | −0.62 | 0.38 | 0.03 |
| Orx2R | SON | Maternal Care | CON+ELSS | 0.37 | 0.14 | 0.04 |
| Orx2R | SON | Maternal Anxiety | CON+ELSS | −0.38 | 0.14 | 0.04 |
| Orx2R | SON | Maternal Anxiety | ELSS | −0.52 | 0.27 | 0.04 |
AVP = Arginine vasopressin, ERK = Extracellular signal-regulated kinase, GHR = Ghrelin receptor, GR = glucocorticoid receptor, MR = mineralocorticoid receptor, Orx1R = Orexin type 1 receptor, Orx2R = Orexin type 2 receptor.
When correlating gene expression levels with behavioral measures, AVP in the CeA was negatively correlated with maternal anxiety in the ECSS dams; in contrast, AVP in the MeA was positively correlated with maternal anxiety. AVP in the PVN was positively correlated with maternal care and negatively correlated with maternal anxiety in both groups combined. ERK protein levels in the NAc were positively correlated with maternal care and negatively correlated with maternal anxiety in both groups combined. Ghrelin R expression in the PVN was positively correlated with maternal anxiety in both groups combined. GR in the SON was negatively correlated with maternal care in control dams, and negatively correlated with maternal anxiety in stressed dams. MR in the PVN was positively correlated with maternal anxiety in both groups combined. Orexin A and Orx1R in the SON were negatively correlated with maternal care in the control dams, and Orx1R and Orx2R were negatively correlated with maternal anxiety in the CSS group or both groups combined. Orx2R was also positively correlated with maternal care in the SON.
DISCUSSION
The present study reports novel changes in gene expression and ERK protein levels in the brains of rat dams exposed to chronic ECSS. Substantial changes in vasopressin, oxytocin, orexin, ghrelin, glucocorticoid and mineralocorticoid receptors, and the BDNF pathway underscore related work on the adverse long-term effects of early life stress on neural activity and plasticity, maternal behavior, responses to stress, and depression and anxiety-related behavior. Correlations between gene targets and both groups combined (as found with AVP, ERK, GHR, MR, and Orx2R) indicate that those gene targets mediate the behavioral difference between the two groups. Correlations with only the control or ECSS groups indicate that the changes in those genes mediate behavioral variation within the control or ECSS group, but that the changes are not directly associated with the between group differences or variation in the other group. The gene and protein expression and robust behavioral correlations support increased focus on vasopressin, ghrelin, orexin and changes in both glucocorticoid and mineralocorticoid receptors in both animal and clinical studies of the adverse effects of early life stress, especially those focusing on depression and anxiety in mothers and transgenerational effects on offspring (Apter-Levy et al., 2013; Feldman et al., 2009; Whelan et al., 2015).
Several studies have confirmed the importance of AVP in the display of maternal care (Bosch and Neumann, 2008; Bosch and Neumann, 2012; Nephew and Murgatroyd, 2013; Nephew, 2012), and the decrease in AVP in the PVN reported here may mediate the previously documented depressed maternal care and increased anxiety in these F1 dams (Carini and Nephew, 2013), as supported by the positive correlations between maternal care and AVP and negative correlations between maternal anxiety and AVP. Neural AVP promotes ongoing maternal care (Bosch and Neumann, 2008; Nephew and Bridges, 2008b), and the central blockage of AVP V1a receptors at parturition interferes with maternal memory (Nephew and Bridges, 2008a). In CSS exposed F0 dams, exogenous chronic icv AVP treatment ameliorates some of the negative effects of social stress on maternal care (Coverdill et al., 2012). Early life exposure of the F1 dams to depressed maternal care and social conflict may decrease PVN AVP activity through a developmental, possibly epigenetic, mechanism which mediates the impaired maternal care of F1 animals towards their F2 pups (Murgatroyd et al., 2009). It is also possible that this change in hypothalamic AVP may mediate the depressed milk intake in the F1 dams, as both maternal care and lactation are decreased in these dams (Carini and Nephew, 2013), similar to comorbid depression and lactational difficulties in humans (Stuebe et al., 2013; Stuebe et al., 2012). In addition to the change in PVN AVP, the expression of this neuropeptide was also altered in the CeA and MeA, and these changes were associated with maternal anxiety on day 2. While the specific functions of the reported changes in amygdalar AVP require further study, it is clear that exposure to ECSS disrupts AVP activity in the brain, and underscores the complex relationship between AVP, maternal behavior, and anxiety (Bosch, 2011; Bosch and Neumann, 2008; Kessler et al., 2011). It is possible that changes in amygdalar AVP were compensatory responses to the increase in maternal anxiety in the ECSS dams, and this hypothesis is supported by the observed changes in expression and correlations between AVP and anxiety. Decreased AVP in the MeA is correlated with maternal anxiety and increased AVP in the CeA is negatively correlated with maternal anxiety.
The other significant change in gene expression in the CeA of stressed dams was an increase in OXTR. Similar to the increase in AVP expression, this change may have been part of compensatory mechanism in response to low levels of OXT in the amygdala and/or deficient maternal care. These data add to recent studies implicating disruption in peripheral and central OXT in pathological differences in depressed mothers (Kim et al., 2014) as well as the growing literature on the role of central OXT in the effects of early social environment on the development of social behavior (Alves et al., 2015). The lack of significant correlations between the neural changes and maternal behavior in the present study may indicate that the effects of ECSS on maternal care and maternal anxiety may be mediated by a complex array of factors and that future rodent and human studies of oxytocin and maternal care should include additional factors associated with OXT. Oxytocin's beneficial or adverse effects may be mediated through changes in ERK mediated plasticity, AVP, corticosteroid receptors, ghrelin, and/or orexin. Taken together, the hypothalamic and amygdalar AVP and OXT findings support the specific importance of these neuropeptides in the regulation of both maternal care and anxiety in animal models of early life stress-associated disorders.
Exposure to ECSS also increases ghrelin R expression in the PVN and this change was correlated with anxiety in both groups combined, similar to reports in male rats (Carlini et al., 2002; Carlini et al., 2004). Chronic icv ghrelin treatment increases depression and anxiety related behaviors in male rats (Hansson et al., 2011), and the PVN appears to be an area particularly sensitive to the anxiogenic effects of ghrelin (Currie et al., 2012), possibly mediated through changes in AVP (Poretti et al., 2015). Serum ghrelin levels in humans are elevated in patients with major depression, and responders to treatments for depression and panic disorder have lower ghrelin levels than non-responders (Ishitobi et al., 2012). On the other hand, ghrelin is reported to mediate resilience to chronic social stress in male mice (Lutter et al., 2008). While more data on developmental changes in peripheral and central ghrelin and ghrelin receptor levels are needed, the current data support the hypothesis that increased ghrelin activity increases maternal anxiety associated behaviors in the ECSS model.
Another HPA-related change in the PVN was an increase in PVN MR expression. ECSS increased MR expression in the PVN and lowered the GR/MR ratio, and MR was correlated with maternal anxiety, a maladaptive change in the ECSS group. There is growing evidence of a role for MR activity in the effects of early life stress on HPA development and activity (Baes et al., 2014; Juruena, 2013; Qi et al., 2013; Young et al., 2003). MR haplotypes mediate the cumulative effects of stress on depression symptoms in females (Klok et al., 2011b), and region dependent differences in neural MR gene expression have been reported in humans (Klok et al., 2011a). The present MR data indicate that social stress exposure exerts its long term effects through changes in hypothalamic MR activity, and the correlation between MR in the PVN and anxiety in both groups combined indicates that MR in this region mediates the effect of ECSS on maternal anxiety. Previous studies of the CSS model support the hypothesis that the reported modulation in the GR/MR ratio is disruptive to the HPA axis. Since it has been documented that F1 animals have elevated basal corticosterone at both the adult and dam stages (Carini and Nephew, 2013), it is postulated that the increase in PVN MR is in response to increased corticosterone levels and/or reduced GR activity. The present MR data support growing interest in central MR function as a novel treatment target for stress associated psychiatric disorders (Harris et al., 2013; Medina et al., 2013; Otte et al., 2015; Von Werne Baes et al., 2012).
The orexin system has been implicated in the pathophysiology of depression (Nollet and Leman, 2013) due to its involvement in the mediation of multiple systems, including arousal, sleep/wake cycles, feeding, stress responses, and reward (Di Sebastiano and Coolen, 2012; Li et al., 2014) and depression associated hypothalamic changes in rodent models (Nocjar et al., 2012). Studies in mice have implicated central orexin activity in the control of maternal care, a robust reward mediated behavior, and maternal aggression (D'Anna and Gammie, 2006). Changes in orexin are also associated with exposure to neonatal maternal deprivation (a robust form of early life stress), and exercise (Feng et al., 2007; James et al., 2014), supporting the hypothesis that orexin may mediate the reported transgenerational effects of ECSS on F1 and F2 offspring. We report hypothalamic changes in orexin activity and depressed maternal care and increased anxiety in dams exposed to ECSS, similar to our previous finding of altered Orx1R expression in the stressed F0 mothers of these F1 animals (Murgatroyd et al., 2015). There was an overall decrease in orexin A activity in the SON, and the associations between Orx2R and maternal care and anxiety in both groups indicates that the ECSS induced changes in behavior are most likely to be mediated by Orx2R, with orexin A and Orx1R being involved in variation in typical maternal care and the individual anxiety response to early life stress. The importance of the orexin receptors in behavioral despair has been previously documented in studies of knock out mice, where an Orx2R knockout displayed increased behavioral despair, with opposite effects in a Orx1R knockout (Scott et al., 2011). Similarly, we report a positive correlation between Orx2R and maternal care, and negative correlation between Orx1R and maternal care. Our data also specifically support recent work reporting decreased hypothalamic orexin A activity in rats exposed to early life maternal separation stress over days 2-14 of lactation (James et al., 2014), another ethologically relevant social stress.
Results from the total ERK and pERK analyses reveal that ERK activity was altered in the ECSS dams compared to controls. While the literature on BDNF and depression and anxiety disorders is mixed, the current results add support to the hypothesis that early life stress alters mechanisms of neuronal plasticity. Despite a decrease in total ERK in the NAc, there was a significant increase in pERK relative to tERK and a trend for increased pERK relative to control protein, which is indicative of greater functional ERK activity. Although we did not see significant effects of ECSS on BDNF levels, it is possible that we missed a significant change in BDNF during or shortly after the ECSS exposure which may have had a lasting organizational or epigenetic effects on downstream targets throughout the CNS. The decrease in total ERK may be indicative of attenuated synaptic plasticity and associated behavioral changes (Marsden, 2013). Similar to the current data, total ERK1/2 protein levels are decreased in the brains of suicide victims (Dwivedi et al., 2001), and it is postulated that this decrease in the stressed dams is an indicator of depressed maternal care. In terms of pERK, social defeat, another potent social stressor commonly used in males, increases pERK in the VTA (Iniguez et al., 2010) and the NAc, and inhibition of ERK signaling in the NAc blocks the effects of social defeat on depression-like behavior in males (Krishnan et al., 2007a). The relative increase in pERK may mediate the effects of ECSS on maternal care and anxiety. The strength of the associations between ERK levels and maternal care and anxiety could indicate that changes in the expression of other behaviorally significant neural systems (AVP, OXT, orexin, corticosterone receptors) are developmentally mediated by alterations in neuroplasticity in the NAc. Given the importance of plasticity in the maternal brain (Galea et al., 2014; Kim et al., 2010; Kinsley and Lambert, 2008), changes in ERK may be especially relevant to peripartum depression and anxiety. While the current study is limited to the NAc, it is possible that ERK-related gene expression is altered in several other regions, such as the hypothalamus and amygdala.
The main limitation of the study is the 20 day interval between the behavioral data and the tissue sampling for gene expression and protein levels. Considering that the exposure to ECSS was during the first two weeks of life of the dams and that the control and ECSS dams were treated identically during the time when they were caring for their own pups, the focus of the present study was on the effects of ECSS on gene expression and behavior that would be expected to be present throughout lactation. While tissue sampling at different time points during lactation would be a valuable component to future studies, prior investigations report that OXT and AVP mRNA and their respective receptor mRNA levels (Nephew et al., 2009) and OXT receptor and AVP V1a receptor binding (Caughey et al., 2011) are relatively consistent across lactation in the PVN, SON, MeA, and CeA of primiparous animals. Furthermore, we focused on targets that are associated with maternal care and/or depression/anxiety both during and not during episodes of impaired maternal care and depression/anxiety, such as plasma oxytocin levels during pregnancy and postpartum depression (Skrundz et al., 2011) and the relationship between early life stress, GR/MR, and depression (Von Werne Baes et al., 2012). Taken together, the design of the CSS model and the focal endocrine targets support the hypothesis that the correlations between behavior during early lactation and gene expression at the end of lactation are relevant for the purpose of identifying factors that may mediate both typical maternal care and the adverse effects of ECSS on maternal care, depression, and anxiety.
With growing evidence for stress associated transgenerational mechanisms and mediating roles of early life stress and/or parental behavior in several psychiatric disorders, the current results on long-term alterations in gene expression, protein levels, and robust behavioral associations support continued or additional translational investigation of the roles of vasopressin, oxytocin, orexin, ghrelin, corticosteroid receptors, and neuroplasticity in stress related disorders in mothers and their offspring. Additional investigations of etiological plasticity in the hypothalamus and amygdala, as well as behavioral gene expression in the nucleus accumbens, are warranted. A combination of targets that already have clinically available treatments (often developed for non-psychiatric conditions) and the preventative potential for future generations (Babb et al., 2015) suggest that research in these areas may be highly productive in the development of new treatments and preventative measures.
Highlights.
Early life social stress altered gene expression in several novel translational targets.
Early life social stress altered BDNF associated indicators of neural plasticity.
These changes were correlated with robust changes in maternal care and anxiety.
Increased focus on vasopressin, ghrelin, and orexin in depression studies is warranted.
ACKNOWLEDGEMENTS
We would like to thank the Tufts University Cummings School Laboratory Animals Medicine Service for outstanding animal care. Gavin Nephew assisted with data collection. This project was funded by NICHD R00 HD HD059943 and a Tufts CTSI Catalyst grant NIH CTSA UL1 TR001064 to BCN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilera M, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H, van Os J, Ibáñez MI, Ruipérez MA, Ortet G, Fañanás L. Early adversity and 5-HTT/BDNF genes: new evidence of gene–environment interactions on depressive symptoms in a general population. Psychological Medicine. 2009;39:1425–1432. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- Alvarez-Crespo M, Skibicka KP, Farkas I, Molnár CS, Egecioglu E, Hrabovszky E, Liposits Z, Dickson SL. The Amygdala as a Neurobiological Target for Ghrelin in Rats: Neuroanatomical, Electrophysiological and Behavioral Evidence. PLoS ONE. 2012;7:e46321. doi: 10.1371/journal.pone.0046321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves E, Fielder A, Ghabriel N, Sawyer M, Buisman-Pijlman FTA. Early Social Environment Affects the Endogenous Oxytocin System: A Review and Future Directions. Frontiers in Endocrinology. 2015;6:32. doi: 10.3389/fendo.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apter-Levy Y, Feldman M, Vakart A, Ebstein RP, Feldman R. Impact of Maternal Depression Across the First 6 Years of Life on the Child's Mental Health, Social Engagement, and Empathy: The Moderating Role of Oxytocin. Am J Psychiatry. 2013 doi: 10.1176/appi.ajp.2013.12121597. [DOI] [PubMed] [Google Scholar]
- Arendt DH, Ronan PJ, Oliver KD, Callahan LB, Summers TR, Summers CH. Depressive behavior and activation of the orexin/hypocretin system. Behavioral Neuroscience. 2013;127:86–94. doi: 10.1037/a0031442. [DOI] [PubMed] [Google Scholar]
- Babb JA, Carini LM, Spears SL, Nephew BC. Transgenerational effects of social stress on social behavior, corticosterone, oxytocin, and prolactin in rats. Horm Behav. 2014;65:386–393. doi: 10.1016/j.yhbeh.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb JA, Deligiannidis KM, Murgatroyd CA, Nephew BC. Peripartum depression and anxiety as an integrative cross domain target for psychiatric preventative measures. Behav Brain Res. 2015 doi: 10.1016/j.bbr.2014.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baes C, Martins CM, Tofoli SM, Juruena MF. Early Life Stress in Depressive Patients: HPA Axis Response to GR and MR Agonist. Front Psychiatry. 2014;5 doi: 10.3389/fpsyt.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Tschöp M, Robinson SM, Heiman ML. Extent and Direction of Ghrelin Transport Across the Blood-Brain Barrier Is Determined by Its Unique Primary Structure. Journal of Pharmacology and Experimental Therapeutics. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Bettscheider M, Murgatroyd C, Spengler D. Simultaneous DNA and RNA isolation from brain punches for epigenetics. BMC Research Notes. 2011;4:314. doi: 10.1186/1756-0500-4-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ. Maternal nurturing is dependent on her innate anxiety: the behavioral roles of brain oxytocin and vasopressin. Hormones and Behavior. 2011;59:202–12. doi: 10.1016/j.yhbeh.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams' trait anxiety. PNAS. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Hormones and Behavior. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Bouma EMC, Ormel J, Verhulst FC, Oldehinkel AJ. Stressful life events and depressive problems in early adolescent boys and girls: The influence of parental depression, temperament and family environment. Journal of Affective Disorders. 2008;105:185–193. doi: 10.1016/j.jad.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Bureau J-F, Easterbrooks MA, Lyons-Ruth K. Maternal depressive symptoms in infancy: Unique contribution to children's depressive symptoms in childhood and adolescence? Development and Psychopathology. 2009;21:519–537. doi: 10.1017/S0954579409000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carini LM, Murgatroyd CA, Nephew BC. Using chronic social stress to model postpartum depression in lactating rodents. J Vis Exp. 2013:e50324. doi: 10.3791/50324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carini LM, Nephew BC. Effects of early life social stress on endocrinology, maternal behavior, and lactation in rats. Hormones and Behavior. 2013;64:634–641. doi: 10.1016/j.yhbeh.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlini VP, Monzón M. a. E., Varas MM, Cragnolini AB, Schiöth HB, Scimonelli TN, de Barioglio SR. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochemical and Biophysical Research Communications. 2002;299:739–743. doi: 10.1016/s0006-291x(02)02740-7. [DOI] [PubMed] [Google Scholar]
- Carlini VP, Varas MM, Cragnolini AB, Schiöth HB, Scimonelli TN, de Barioglio SR. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochemical and Biophysical Research Communications. 2004;313:635–641. doi: 10.1016/j.bbrc.2003.11.150. [DOI] [PubMed] [Google Scholar]
- Carter CS. Developmental consequences of oxytocin. Physiology & Behavior. 2003;79:383–397. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- Caughey SD, Klampfl SM, Bishop VR, Pfoertsch J, Neumann ID, Bosch OJ, Meddle SL. Changes in the Intensity of Maternal Aggression and Central Oxytocin and Vasopressin V1a Receptors Across the Peripartum Period in the Rat. Journal of Neuroendocrinology. 2011;23:1113–1124. doi: 10.1111/j.1365-2826.2011.02224.x. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Coverdill A, McCarthy M, Bridges R, Nephew B. Effects of Chronic Central Arginine Vasopressin (AVP) on Maternal Behavior in Chronically Stressed Rat Dams. Brain Sciences. 2012;2:589–604. doi: 10.3390/brainsci2040589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie PJ, Khelemsky R, Rigsbee EM, Dono LM, Coiro CD, Chapman CD, Hinchcliff K. Ghrelin is an orexigenic peptide and elicits anxiety-like behaviors following administration into discrete regions of the hypothalamus. Behavioural Brain Research. 2012;226:96–105. doi: 10.1016/j.bbr.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Anna KL, Gammie SC. Hypocretin-1 dose-dependently modulates maternal behaviour in mice. Journal of Neuroendocrinology. 2006;18:553–66. doi: 10.1111/j.1365-2826.2006.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sebastiano AR, Coolen LM. Orexin and natural reward: feeding, maternal, and male sexual behavior. Prog Brain Res. 2012;198:65–77. doi: 10.1016/B978-0-444-59489-1.00006-9. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Duric V, Banasr M, Licznerski P, Schmidt H, Stockmeier C. A negative regulator of MAP kinase causes depressive behavior. Nat. Med. 2010;16:1328. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, Tamminga CA, Pandey GN. Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. Journal of neurochemistry. 2001;77:916–928. doi: 10.1046/j.1471-4159.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- Eiland L, McEwen BS. Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus. 2012;22:82–91. doi: 10.1002/hipo.20862. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, Kalin NH, Armstrong JM. Influence of early life stress on later hypothalamic–pituitary–adrenal axis functioning and its covariation with mental health symptoms: A study of the allostatic process from childhood into adolescence. Development and Psychopathology. 2011;23:1039–1058. doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faron-Górecka A, Kuśmider M, Kolasa M, Żurawek D, Gruca P, Papp M, Szafran K, Solich J, Pabian P, Romańska I, Antkiewicz-Michaluk L, Dziedzicka-Wasylewska M. Prolactin and its receptors in the chronic mild stress rat model of depression. Brain research. 2014;1555:48–59. doi: 10.1016/j.brainres.2014.01.031. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent–infant synchrony: considering stress and affiliation components of human bonding. Developmental Science. 2011;14:752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:919–27. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Feng P, Vurbic D, Wu Z, Strohl KP. Brain orexins and wake regulation in rats exposed to maternal deprivation. Brain Res. 2007;18:163–72. doi: 10.1016/j.brainres.2007.03.077. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Leuner B, Slattery DA. Hippocampal Plasticity during the Peripartum Period: Influence of Sex Steroids, Stress and Ageing. Journal of Neuroendocrinology. 2014;26:641–648. doi: 10.1111/jne.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, Gordon E, Kemp AH, Williams LM. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009;14:681–695. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Goodman S, Rouse M, Connell A, Broth M, Hall C, Heyward D. Maternal depression and child psychopathology: A meta-analytic review. Clinical Child and Family Psychology Review. 2011;14:1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- Goodman SH. Depression in Mothers. Annu. Rev. Clin. Psychol. 2007;3:107–35. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, Taylor JR. Regionally Specific Regulation of ERK MAP Kinase in a Model of Antidepressant-Sensitive Chronic Depression. Biological Psychiatry. 2008;63:353–359. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. Stress neurobiology and developmental psychopathology. In: Cohen DCDJ, editor. Developmental psychopathology, Vol 2: Developmental neuroscience. 2nd ed. John Wiley & Sons Inc; Hoboken, NJ, US: 2006. pp. 533–577. [Google Scholar]
- Hansson C, Haage D, Taube M, Egecioglu E, Salomé N, Dickson SL. Central administration of ghrelin alters emotional responses in rats: behavioural, electrophysiological and molecular evidence. Neuroscience. 2011;180:201–211. doi: 10.1016/j.neuroscience.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Harris AP, Holmes MC, de Kloet ER, Chapman KE, Seckl JR. Mineralocorticoid and glucocorticoid receptor balance in control of HPA axis and behaviour. Psychoneuroendocrinology. 2013;38:648–658. doi: 10.1016/j.psyneuen.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Experimental Neurology. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Owens MJ, Plotsky PM, Nemeroff CB. Persistent changes in corticotropin-releasing factor systems due to early life stress: Relationship to the pathophysiology of major depression and post-traumatic stress disorder. Psychopharmacology Bulletin. 1997;33:185–192. [PubMed] [Google Scholar]
- Henriques TP, Szawka RE, Diehl LA, de Souza MA, Corrêa CN, Aranda BCC, Sebben V, Franci CR, Anselmo-Franci JA, Silveira PP, de Almeida RMM. Stress in Neonatal Rats with Different Maternal Care Backgrounds: Monoaminergic and Hormonal Responses. Neurochemical Research. 2014;39:2351–2359. doi: 10.1007/s11064-014-1434-8. [DOI] [PubMed] [Google Scholar]
- Iniguez SD, Vialou V, Warren BL, Cao JL, Alcantara LF, Davis LC, Manojlovic Z, Neve RL, Russo SJ, Han MH, Nestler EJ, Bolanos-Guzman CA. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J. Neurosci. 2010;30:7652–63. doi: 10.1523/JNEUROSCI.0951-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Ishitobi Y, Kohno K, Kanehisa M, Inoue A, Imanaga J, Maruyama Y, Ninomiya T, Higuma H, Okamoto S, Tanaka Y, Tsuru J, Hanada H, Isogawa K, Akiyoshi J. Serum Ghrelin Levels and the Effects of Antidepressants in Major Depressive Disorder and Panic Disorder. Neuropsychobiology. 2012;66:185–192. doi: 10.1159/000339948. [DOI] [PubMed] [Google Scholar]
- James MH, Campbell EJ, Walker FR, Smith DW, Richardson HN, Hodgson DM, Dayas CV. Exercise reverses the effects of early life stress on orexin cell reactivity in male but not female rats. Frontiers in Behavioral Neuroscience. 2014;8 doi: 10.3389/fnbeh.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanneteau F, Deinhardt K, Miyoshi G, Bennett AM, Chao MV. The MAP kinase phosphatase MKP-1 regulates BDNF-induced axon branching. Nat Neurosci. 2010;13:1373–9. doi: 10.1038/nn.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JH, Sarason IG. Life stress, depression and anxiety: Internal-external control as a moderator variable. Journal of Psychosomatic Research. 1978;22:205–208. doi: 10.1016/0022-3999(78)90025-9. [DOI] [PubMed] [Google Scholar]
- Juruena MF. Early-life stress and HPA axis trigger recurrent adulthood depression. Epilepsy Behav. 2013;19:00553–2. doi: 10.1016/j.yebeh.2013.10.020. [DOI] [PubMed] [Google Scholar]
- Juruena MF, Pariante CM, Papadopoulos AS, Poon L, Lightman S, Cleare AJ. The role of mineralocorticoid receptor function in treatment-resistant depression. Journal of Psychopharmacology. 2013 doi: 10.1177/0269881113499205. [DOI] [PubMed] [Google Scholar]
- Kessler MS, Bosch OJ, Bunck M, Landgraf R, Neumann ID. Maternal care differs in mice bred for high vs. low trait anxiety: impact of brain vasopressin and cross-fostering. Social Neuroscience. 2011;6:156–68. doi: 10.1080/17470919.2010.495567. [DOI] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE. The plasticity of human maternal brain: Longitudinal changes in brain anatomy during the early postpartum period. Behavioral Neuroscience. 2010;124:695–700. doi: 10.1037/a0020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Soeken TA, Cromer SJ, Martinez SR, Hardy LR, Strathearn L. Oxytocin and postpartum depression: Delivering on what's known and what's not. Brain research. 2014;1580:219–232. doi: 10.1016/j.brainres.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsley CH, Lambert KG. Reproduction-Induced Neuroplasticity: Natural Behavioural and Neuronal Alterations Associated with the Production and Care of Offspring. Journal of Neuroendocrinology. 2008;20:515–525. doi: 10.1111/j.1365-2826.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- Klok MD, Alt SR, Irurzun Lafitte AJM, Turner JD, Lakke EAJF, Huitinga I, Muller CP, Zitman FG, Ronald de Kloet E, DeRijk RH. Decreased expression of mineralocorticoid receptor mRNA and its splice variants in postmortem brain regions of patients with major depressive disorder. Journal of Psychiatric Research. 2011a;45:871–878. doi: 10.1016/j.jpsychires.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Klok MD, Giltay EJ, Van der Does AJW, Geleijnse JM, Antypa N, Penninx BWJH, de Geus EJC, Willemsen G, Boomsma DI, van Leeuwen N, Zitman FG, de Kloet ER, DeRijk RH. A common and functional mineralocorticoid receptor haplotype enhances optimism and protects against depression in females. Transl Psychiatry. 2011b;1:e62. doi: 10.1038/tp.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007a;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007b;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Li J, Hu Z, de Lecea L. The hypocretins/orexins: integrators of multiple physiological functions. British Journal of Pharmacology. 2014;171:332–350. doi: 10.1111/bph.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D. Maternal care, hippocampal glucocorticoid receptors, and the hypothalamic-pituitary-adrenal responses to stress. Nat Neurosci. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lizardi H, Klein DN, Ouimette PC, Riso LP, Anderson RL, Donaldson SK. Reports of the childhood home environment in early-onset dysthymia and episodic major depression. Journal of Abnormal Psychology. 1995;104:132–139. doi: 10.1037//0021-843x.104.1.132. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Pruessner J, Sousa N, Almeida OF, Van Dam AM, Rajkowska G, Swaab DF, Czeh B. Neuropathology of stress. Acta Neuropathol. 2014;127:109–35. doi: 10.1007/s00401-013-1223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon AL, Gold I, Feeley N, Hayton B, Carter CS, Zelkowitz P. The role of oxytocin in mothers’ theory of mind and interactive behavior during the perinatal period. Psychoneuroendocrinology. 2014;48:52–63. doi: 10.1016/j.psyneuen.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Mann PE, Bridges RS. Lactogenic hormone regulation of maternal behavior. Progress in brain research. 2001;133:251–62. doi: 10.1016/s0079-6123(01)33019-4. [DOI] [PubMed] [Google Scholar]
- Marsden WN. Synaptic plasticity in depression: Molecular, cellular and functional correlates. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;43:168–184. doi: 10.1016/j.pnpbp.2012.12.012. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Early life influences on life-long patterns of behavior and health. Mental Retardation and Developmental Disabilities Research Reviews. 2003;9:149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- McGowan P, Sasaki A, D'Alessio A, Dymov S, Labonte B. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12:342. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina A, Seasholtz AF, Sharma V, Burke S, Bunney W, Jr, Myers RM, Schatzberg A, Akil H, Watson SJ. Glucocorticoid and mineralocorticoid receptor expression in the human hippocampus in major depressive disorder. Journal of Psychiatric Research. 2013;47:307–314. doi: 10.1016/j.jpsychires.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan S, Snow S, Belay S. Depressive symptoms in mothers and children: Preschool attachment as a moderator of risk. Developmental Psychology. 2009;45:1019–1033. doi: 10.1037/a0016164. [DOI] [PubMed] [Google Scholar]
- Mogi K, Nagasawa M, Kikusui T. Developmental consequences and biological significance of mother–infant bonding. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35:1232–1241. doi: 10.1016/j.pnpbp.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nature neuroscience. 2009;12:1559–66. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Murgatroyd CA, Nephew BC. Effects of early life social stress on maternal behavior and neuroendocrinology. Psychoneuroendocrinology. 2013;38:219–228. doi: 10.1016/j.psyneuen.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd CA, Taliefar M, Bradburn S, Carini LM, Babb JA, Nephew BC. Social stress during lactation, depressed maternal care, and neuropeptidergic gene expression. Behavioural Pharmacology. 2015 doi: 10.1097/FBP.0000000000000147. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Akiyoshi J, Hatano K, Hanada H, Tanaka Y, Tsuru J, Matsushita H, Kodama K, Isogawa K. Ghrelin gene polymorphism is associated with depression, but not panic disorder. Psychiatric Genetics. 2008;18:257. doi: 10.1097/YPG.0b013e328306c979. 10.1097/YPG.0b013e328306c979. [DOI] [PubMed] [Google Scholar]
- Nephew B, Murgatroyd C. The role of maternal care in shaping CNS function. Neuropeptides. 2013;47:371–378. doi: 10.1016/j.npep.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC. Behavioral roles of oxytocin and vasopressin. In: Sumiyoshi T, editor. Neuroendocrinology and Behavior. InTech; 2012. [Google Scholar]
- Nephew BC, Bridges RS. Arginine vasopressin V1a receptor antagonist impairs maternal memory in rats. Physiology & Behavior. 2008a;95:182–186. doi: 10.1016/j.physbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacology Biochemistry and Behavior. 2008b;91:77–83. doi: 10.1016/j.pbb.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress. 2011;14:677–684. doi: 10.3109/10253890.2011.605487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS, Lovelock DF, Byrnes EM. Enhanced maternal aggression and associated changes in neuropeptide gene expression in multiparous rats. Behavioral Neuroscience. 2009;123:949–57. doi: 10.1037/a0016734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocjar C, Zhang J, Feng P, Panksepp J. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience. 2012;218:138–53. doi: 10.1016/j.neuroscience.2012.05.033. [DOI] [PubMed] [Google Scholar]
- Nollet M, Leman S. Role of orexin in the pathophysiology of depression: potential for pharmacological intervention. CNS Drugs. 2013;27:411–22. doi: 10.1007/s40263-013-0064-z. [DOI] [PubMed] [Google Scholar]
- Otte C, Wingenfeld K, Kuehl LK, Kaczmarczyk M, Richter S, Quante A, Regen F, Bajbouj M, Zimmermann-Viehoff F, Wiedemann K, Hinkelmann K. Mineralocorticoid Receptor Stimulation Improves Cognitive Function and Decreases Cortisol Secretion in Depressed Patients and Healthy Individuals. Neuropsychopharmacology. 2015;40:386–393. doi: 10.1038/npp.2014.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña CJ, Neugut YD, Champagne FA. Developmental Timing of the Effects of Maternal Care on Gene Expression and Epigenetic Regulation of Hormone Receptor Levels in Female Rats. Endocrinology. 2013 doi: 10.1210/en.2013-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello M, Scott MM, Sakata I, Lee CE, Chuang J-C, Osborne-Lawrence S, Rovinsky SA, Elmquist JK, Zigman JM. Functional implications of limited leptin receptor and ghrelin receptor coexpression in the brain. The Journal of Comparative Neurology. 2012;520:281–294. doi: 10.1002/cne.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretti MB, Rask-Andersen M, Kumar P, Rubiales de Barioglio S, Fiol de Cuneo M, Schiöth HB, Carlini VP. Ghrelin effects expression of several genes associated with depression-like behavior. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2015;56:227–234. doi: 10.1016/j.pnpbp.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Qi XR, Kamphuis W, Wang S, Wang Q, Lucassen PJ, Zhou JN, Swaab DF. Aberrant stress hormone receptor balance in the human prefrontal cortex and hypothalamic paraventricular nucleus of depressed patients. Psychoneuroendocrinology. 2013;38:863–70. doi: 10.1016/j.psyneuen.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T, Lubin F, Funk A, Sweatt J. Lasting epigenetic influence of early life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Scott MM, Marcus JN, Pettersen A, Birnbaum SG, Mochizuki T, Scammell TE, Nestler EJ, Elmquist JK, Lutter M. Hcrtr1 and 2 signaling differentially regulates depression-like behaviors. Behavioural Brain Research. 2011;222:289–94. doi: 10.1016/j.bbr.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. Plasma Oxytocin Concentration during Pregnancy is associated with Development of Postpartum Depression. Neuropsychopharmacology. 2011;36:1886–1893. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe AM, Grewen K, Meltzer-Brody S. Association between maternal mood and oxytocin response to breastfeeding. J Womens Health. 2013;22:352–61. doi: 10.1089/jwh.2012.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe AM, Grewen K, Pedersen CA, Propper C, Meltzer-Brody S. Failed lactation and perinatal depression: common problems with shared neuroendocrine mechanisms? Journal of Women's Health. 2012;21:264–72. doi: 10.1089/jwh.2011.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suderman M, McGowan PO, Sasaki A, Huang TCT, Hallett MT, Meaney MJ, Turecki G, Szyf M. Conserved epigenetic sensitivity to early life experience in the rat and human hippocampus. Proceedings of the National Academy of Sciences. 2012 doi: 10.1073/pnas.1121260109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Werne Baes C, de Carvalho Tofoli SM, Martins CMS, Juruena MF. Assessment of the hypothalamic–pituitary–adrenal axis activity: glucocorticoid receptor and mineralocorticoid receptor function in depression with early life stress – a systematic review. Acta Neuropsychiatrica. 2012;24:4–15. doi: 10.1111/j.1601-5215.2011.00610.x. [DOI] [PubMed] [Google Scholar]
- Whelan YM, Leibenluft E, Stringaris A, Barker ED. Pathways from maternal depressive symptoms to adolescent depressive symptoms: the unique contribution of irritability symptoms. Journal of Child Psychology and Psychiatry. 2015 doi: 10.1111/jcpp.12395. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Mineralocorticoid receptor function in major depression. Arch Gen Psychiatry. 2003;60:24–8. doi: 10.1001/archpsyc.60.1.24. [DOI] [PubMed] [Google Scholar]
- Zamorano M, Ledesma-Colunga MG, Adan N, Vera-Massieu C, Lemini M, Mendez I, Moreno-Carranza B, Neumann ID, Thebault S, Martinez de la Escalera G, Torner L, Clapp C. Prolactin-derived vasoinhibins increase anxiety- and depression-related behaviors. Psychoneuroendocrinology. 2014;44:123–32. doi: 10.1016/j.psyneuen.2014.03.006. [DOI] [PubMed] [Google Scholar]